Nickel base catalyst preparation method
A nickel-based catalyst and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem that the catalyst has a small average pore size and is not suitable for the reaction of macromolecular substances and other issues to achieve stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
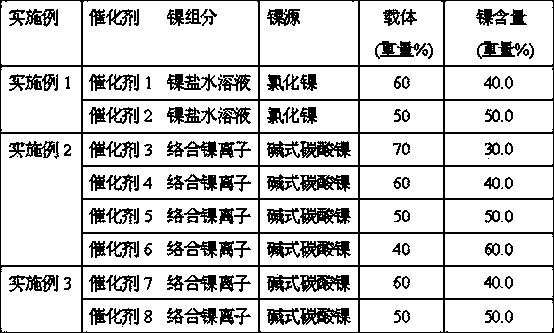
[0016] With nitric acid in [H + ] / [AlOOH] molar ratio is under the condition of 0.25, peptized pseudo-boehmite powder 24 hours, obtains alumina sol 1500g that alumina solid content is 5%, nickel chloride is dissolved in an appropriate amount of water, obtains 0.10 grams of nickel / ml of nickel salt solution. Add an appropriate amount of nickel salt solution to the aluminum sol, adjust the PH~8.5 of the sol with ammonia water, and then o Under aging for 24 hours under C, then through filtration, washing and vacuum drying (vacuum tightness 30KPa, drying time 20 hours, promptly obtain corresponding catalyst precursor. Catalyst precursor is in 400 o C roasting for 4 hours to obtain oxidized NiO / Al 2 o 3 catalyst. Catalyst in 1.5 L / min pure hydrogen flow, at 450 o Reduction at C for 12 hours to obtain metallic Ni / Al 2 o 3 catalyst.
[0017] Change the amount of nickel salt solution added to the aluminum sol to obtain catalysts 1-2 in turn, see Table 1. The physical property...
Embodiment 2
[0020] With nitric acid in [H + ] / [AlOOH] molar ratio of 0.25, the pseudo-boehmite powder was peptized for 24 hours to obtain 1500 g of alumina sol with a solid content of 5% alumina. In terms of molar ratio, basic nickel carbonate: ammonia water: ammonium carbonate = 1: 6.0: 1.5, adding an appropriate amount of water to obtain a nickel-ammonia complex solution of 0.10 g nickel / ml. Add an appropriate amount of nickel ammonia complex solution to the aluminum sol, at 95 o Heat and decompose the complexed nickel ions at C for 8 hours, and then filter, wash and vacuum dry (vacuum degree 50KPa, drying time 25 hours) to obtain the corresponding catalyst precursor. catalyst precursor at 400 o C roasting for 4 hours to obtain oxidized NiO / Al 2 o 3 catalyst. Catalyst in 1.5 L / min pure hydrogen flow, at 450 o Reduction at C for 12 hours to obtain metallic Ni / Al 2 o 3 catalyst.
[0021] Change the amount of nickel-ammonia complex solution added to the aluminum sol to obtain cata...
Embodiment 3
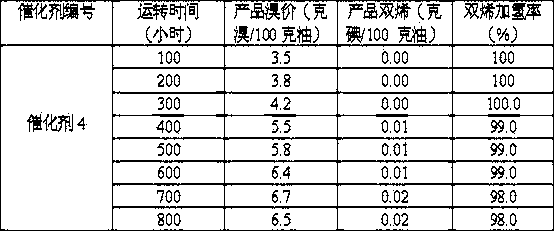
[0031] The hydrogenation activity test and 800-hour stability test of the catalyst of the present invention were carried out. The properties of the raw oil used in the experiment are shown in Table 3. Catalyst 4 in embodiment 2 and catalyst 7 in comparative example 1 were respectively at 450 o C was reduced with hydrogen for 24 hours. The hydrogenation reaction uses an adiabatic fixed-bed reactor, and the process conditions are: the inlet temperature is 50 o C, pressure 3.0 MPa, fresh oil space velocity LHSV=1.5 hours -1 , the volume ratio of hydrogen to oil H 2 / Feed oil = 600:1, the evaluation results of Catalyst 4 and Catalyst 7 are shown in Table 4 and Table 5.
[0032] table 3
[0033]
[0034] Table 4
[0035]
[0036] table 5
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com