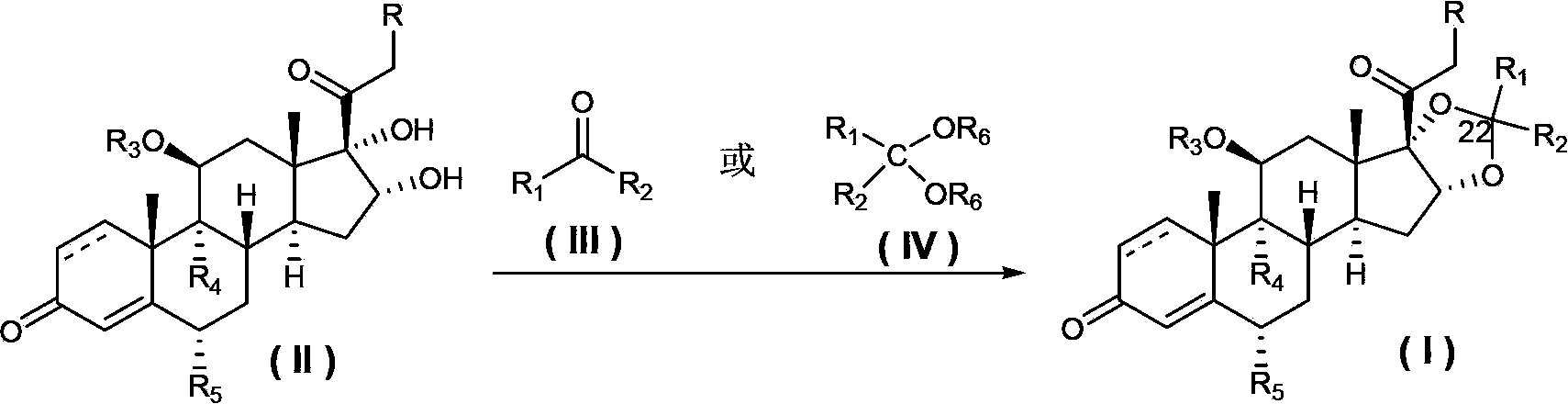
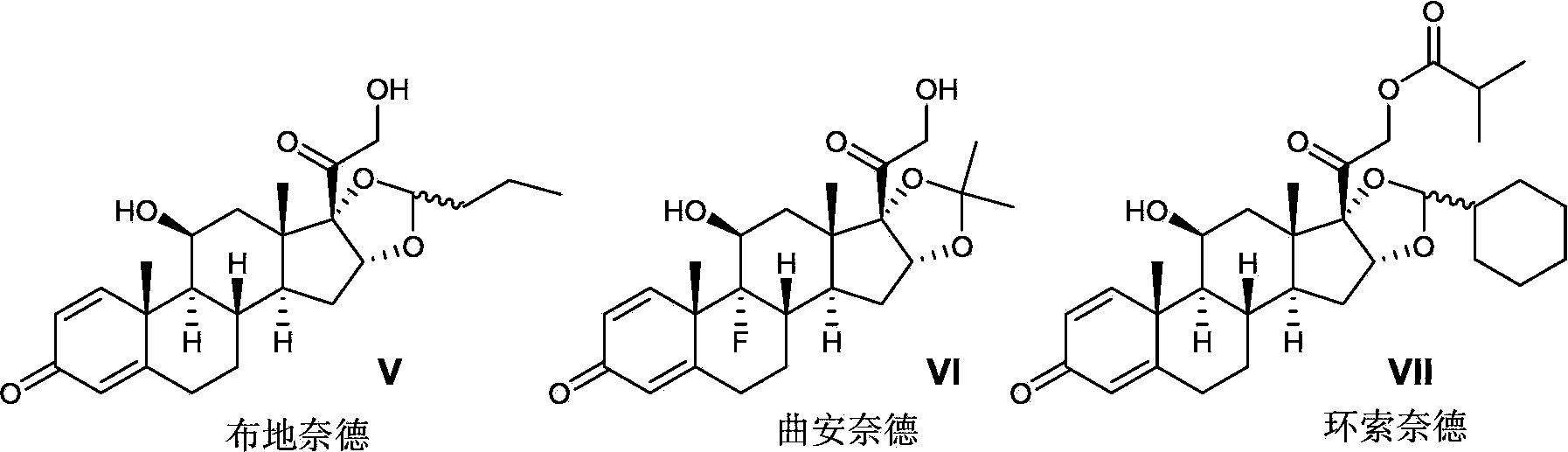
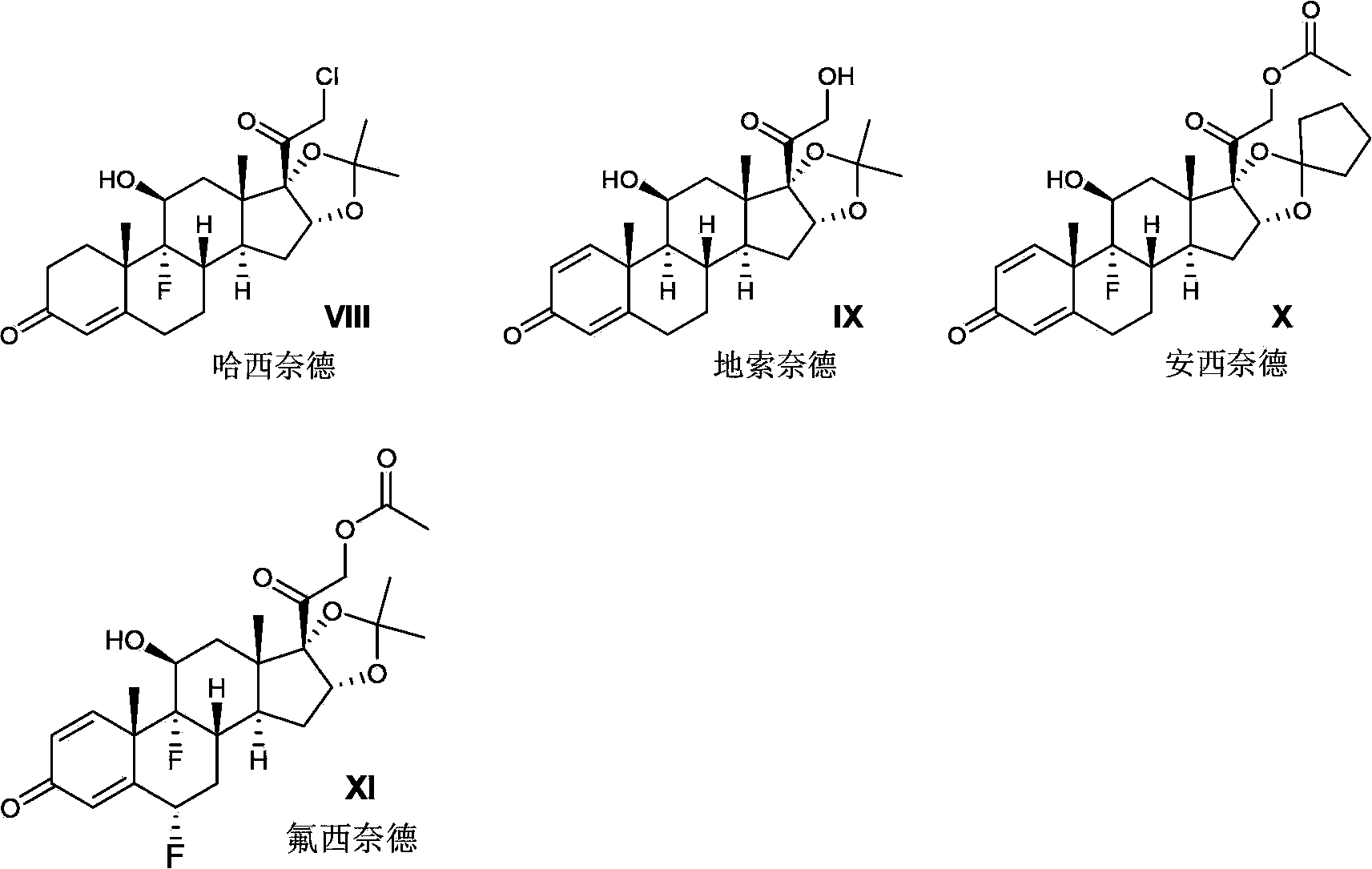
Preparation method of pregnane derivatives 16,17-acetal (ketone)
A derivative, pregnane technology, applied in the field of medicinal chemistry, can solve the problems of many by-products, low selectivity, and large environmental pollution, and achieve the effects of improved quality and yield, mild reaction conditions and significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 budesonide
[0032]
[0033] in N 2 Under protection, 16α-hydroxyprednisolone (II-1) (purchased from Hunan Yuxin Pharmaceutical Co., Ltd.) (50g, 13.3mmol) and 47% boron trifluoride tetrahydrofuran solution (6g) were added into acetonitrile (500ml ), keep the temperature between -5°C and 0°C, slowly drop n-butyraldehyde (III-1) (14g), after the drop, keep the temperature at 0-10°C, and react for 5-6h , Sodium bicarbonate (10g) was dissolved in water (1500ml), the temperature of the aqueous sodium bicarbonate solution was kept at 0-10°C, the above reaction solution was slowly poured in, stirred for 0.5h, filtered and dried to obtain budesinate German crude product 53g, the ratio of 22 carbon R and S is 55:45 (HPLC). Add the above crude product into ethanol (530ml), heat to reflux until all the solids are dissolved, then keep the temperature at 60-70°C, slowly add purified water (1060ml) dropwise, after dropping, slowly lower the temper...
Embodiment 2
[0034] The preparation of embodiment 2 triamcinolone acetonide
[0035]
[0036]Triamcinolone (II-2) (purchased from Sinopharm Chemical Reagent Co., Ltd.) (8g, 20mmol) was mixed with acetone (III-2) (400ml), and slowly added 46.5% of Boron trifluoride ether solution (2ml), react overnight at 25°C, add saturated sodium bicarbonate solution (10ml) under stirring, concentrate the reaction solution to about 50ml, filter the precipitated solid, wash with water, and dry to give triamcinolone acetonide 8.2g, yield : 93%, m.p: 288-290°C.
Embodiment 3
[0037] The preparation of embodiment 3 desonide
[0038]
[0039] in N 2 Under protection, 16α-hydroxyprednisolone (II-1) (5g, 1.33mmol) and 47% boron trifluoride tetrahydrofuran solution (0.6g) were added to acetonitrile (50ml), and the temperature was kept at -5°C Slowly drop in acetone (III-2) (1.2g) between temperature and 0°C. After dropping, keep the temperature at 0-10°C. After reacting for 5-6h, filter the solid and rinse the solid with acetonitrile. Dry to obtain 4.7g of desonide, the yield is 85%, the purity is greater than 99% (HPLC), m.p: 274-275 ℃, 1 H NMR (CDCl 3 ,300MHz)δ0.87(3H,s),1.42(3H,s),1.46(3H,s),2.38(1H,dd),2.6(1H,m),3.1(1H,brs),4.18(1H ,d), 4.52(1H,d), 4.70(1H,d), 5.06(1H,d), 6.02(1H,s), 6.3(1H,dd).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com