Bacteria Lysinibacillus sp. for adsorbing gold and antimony
A bacterial strain and heavy metal technology, applied in the direction of selective adsorption, adsorption of water/sewage treatment, bacteria, etc., can solve the problems of difficulty in popularization and application, damage, strong corrosion and toxicity, and achieve the effect of high application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
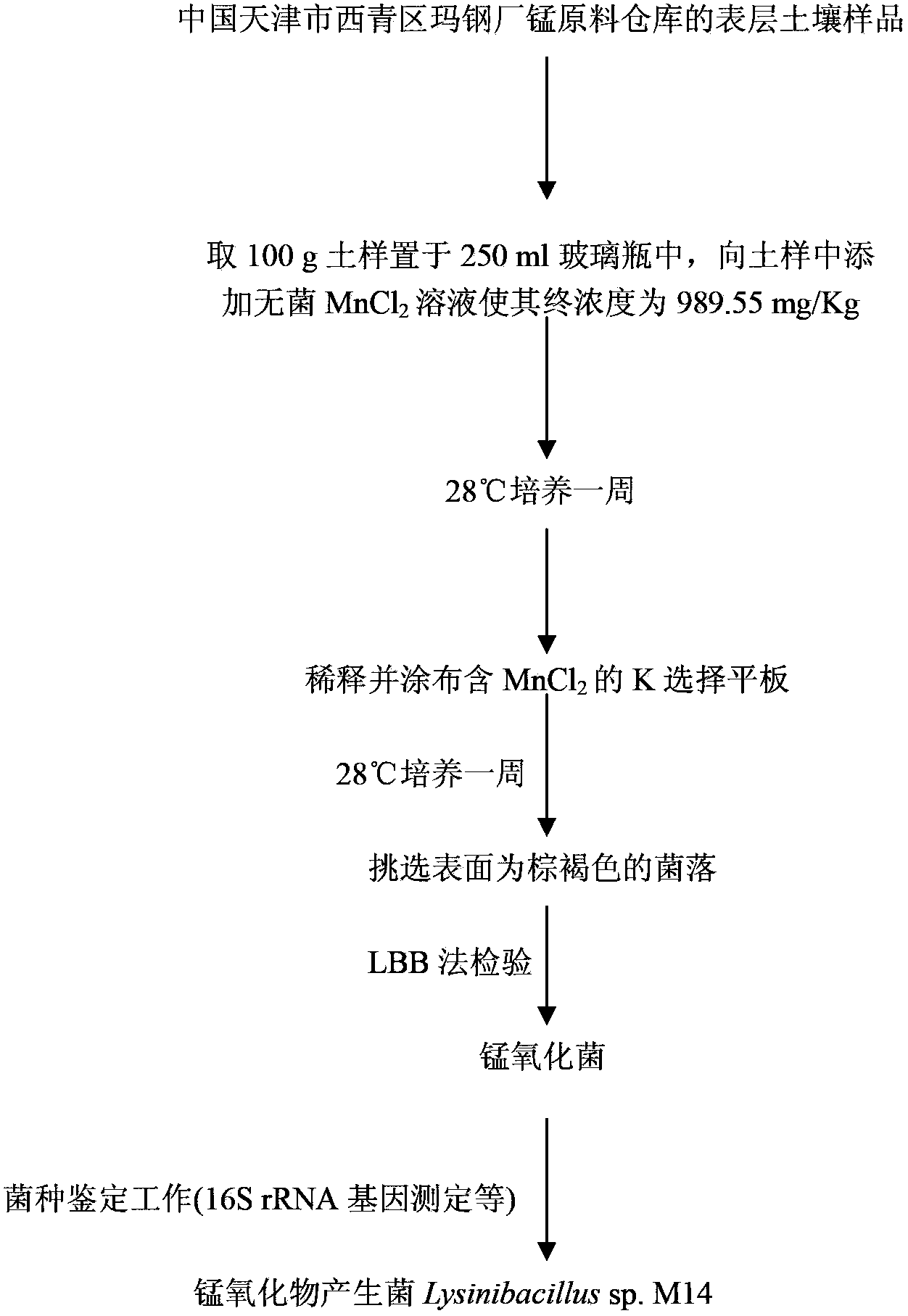
[0023] Example 1: Isolation and identification of Lysinibacillus sp.M14 from manganese-contaminated soil
[0024] (1) Sample collection: In late June 2007, the manganese soil samples for this test were collected from the surface soil of the manganese raw material warehouse of Maanshan Iron and Steel Plant in Xiqing District, Tianjin, China.
[0025] (2) Sample enrichment: take 100g of soil sample, add sterile MnCl to the soil sample 2 The solution was made to have a final concentration of 989.55mg / Kg, stirred gently and placed in an incubator at 28°C for one week, and added sterile water to ensure the humidity of the sample.
[0026] (3) Isolation of manganese oxidizing bacteria: accurately weigh the MnCl 2 Put 10g of the enriched soil sample in a conical flask filled with 90ml of sterile normal saline, shake it in a shaker at 28°C for half an hour, then take 1ml of it and add it to 9ml of sterile normal saline to gradually dilute to 10 -2 , 10 -3 , 10 -4 , take 0.1ml and ...
Embodiment 2
[0034] Example 2: Manganese oxidation removal curve of manganese oxide producing bacteria (Lysinibacillus sp.) M14
[0035] Prepare 3 bottles of sterilized liquid K medium (the formula is the same as above), 100mL in each bottle. Add 20mmol / L hydroxyethylpiperazineethanesulfonic acid (HEPES) that has been filtered and sterilized, and add 1mol / L sterile MnCl at the same time 2 The final concentration of mother liquor is 0.3mmol / L. Then inoculate the logarithmic phase (OD 600 The value is about 0.7) M14 bacteria liquid, inoculated with an inoculum volume of 1% by volume. Immediately after mixing, take a 10mL sample, which is taken as the first sample, and placed in a clean centrifuge tube. Each Erlenmeyer flask was placed at 28°C and shaken at 160r / min for culture. Thereafter, the sampling interval was 24 h, and the experiment was repeated three times. After sampling, the samples were treated as follows:
[0036] 1) 10mL samples obtained each time were centrifuged at 8000r...
Embodiment 3
[0041] Example 3: Determination of the ability of Lysinibacillus sp.M14 to produce manganese oxides to adsorb gold in gold-containing solutions
[0042] Lysinibacillus sp.M14 thalline of the present invention is inoculated in containing 5mM Mn 2+ In the K medium (formulation as above), culture in a shaker at 28°C and 150r / min for 10 days, the total amount of the medium is 3000mL, and after brown-black precipitates are formed in the medium, culture at a high speed of 8000r / min The supernatant was discarded, and the precipitate was freeze-dried at -56°C, ground, and passed through a 100-mesh sieve. Add 1.87g of biomanganese oxide to a conical flask filled with 100mL of double-distilled water. Before the experiment, the conical flask should be covered with black plastic to prevent Au(III) from changing color when exposed to light. After shaking well, add 200μL of 100mmol / L chloroauric acid, after mixing, sample 3mL as the first sampling. Place each Erlenmeyer flask in a shaker ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com