Drug composition for treating vomiting during pregnancy
A composition and drug technology, applied in the field of medicine, can solve the problem of too many pharmaceutical excipients, achieve large-scale production, facilitate absorption, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
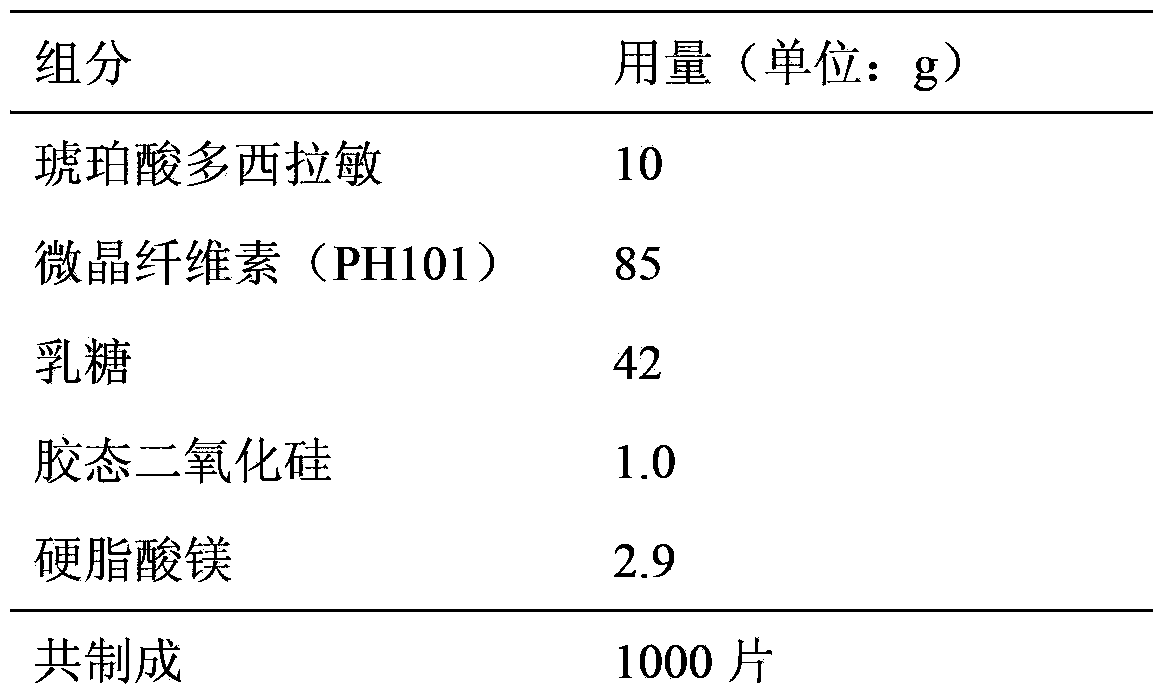
Embodiment 1
[0013]
[0014] Preparation:
[0015] (1) Pass doxylamine succinate through an 80-mesh sieve and set aside;
[0016] (2) Weigh the prescribed amount of microcrystalline cellulose (PH101) and lactose and mix them evenly;
[0017] (3) Water-made soft material, granulated with 24 mesh, dried at 60°C, and granulated with 40 mesh;
[0018] (4) Add the prescribed amount of magnesium stearate and colloidal silicon dioxide, and mix well;
[0019] (5) Intermediate detection and content determination.
[0020] (6) Calculate the tablet weight based on the content of the semi-finished product, adjust the tablet weight and pressure and then press the tablet.
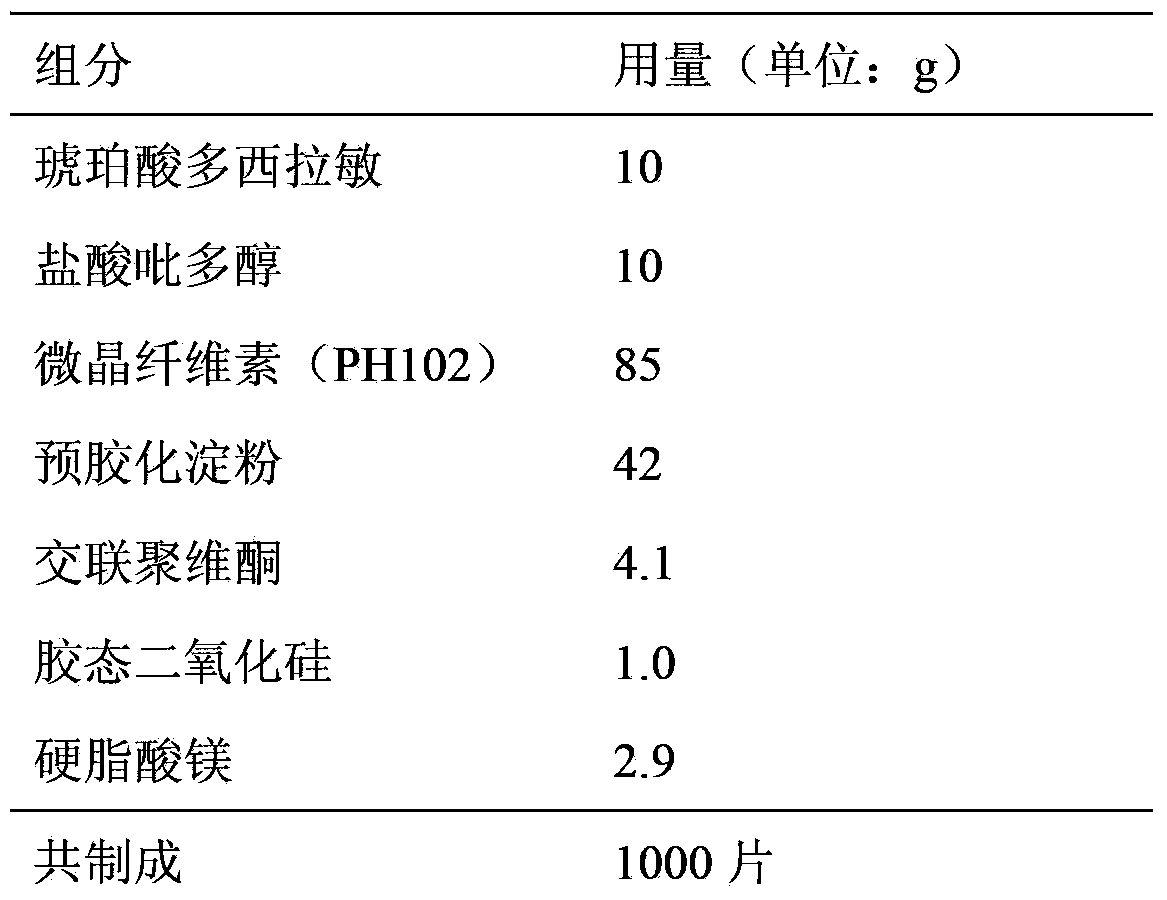
Embodiment 2
[0022] prescription:
[0023]
[0024] Preparation:
[0025] (1) Pass doxylamine succinate and pyridoxine hydrochloride through an 80-mesh sieve and set aside;
[0026] (2) Weigh the prescribed amount of microcrystalline cellulose (PH102), pregelatinized starch, crospovidone, colloidal silicon dioxide, magnesium stearate and the main drug, and mix them evenly;
[0027] (3) The powder is directly pressed into tablets, acquired;
[0028] Because pyridoxine hydrochloride is easy to decompose when exposed to light, and the main part of the human body is the jejunum, the obtained plain tablets are coated with an enteric coating solution to ensure the safety of the medicine for long-term storage and avoid the destruction of gastric acid.
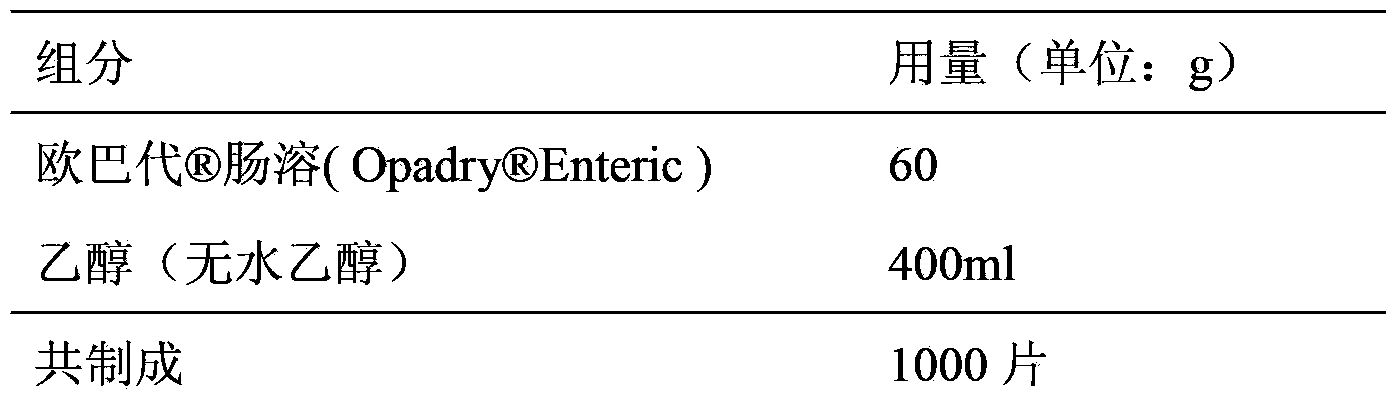
[0029] Coating liquid formula:
[0030]
[0031] (1) Take the prescribed amount of coating powder, slowly add it to the 50% ethanol solution under constant stirring, continue to stir for about 45-60 minutes, and set aside.
[0032] (2) Put the plain tablets in...
Embodiment 3
[0034] Since the solvent used in the coating liquid is absolute ethanol, considering that the pharmaceutical composition is mainly used for pregnant women, other enteric coating liquids are used instead.
[0035] prescription:
[0036]
[0037] Coating liquid formula:
[0038]
[0039]
[0040] (1) Take the prescribed amount of coating powder, slowly add it to the 50% ethanol solution under constant stirring, continue to stir for about 45-60 minutes, and set aside.
[0041] (2) Put the plain tablets in the coating pan, turn on the exhaust and dust collection device, heat to 40℃, turn on the coating pan, blow off the surface fine powder of the plain tablets, and preheat the plain tablets. Turn on the air compressor, adjust the speed of spraying liquid and air pressure, so that the coating liquid is sprayed in a mist form and sprayed onto the surface of the plain sheet. Continue spraying until the coating requirements are met, and continue hot air drying for 10 minutes. The coating we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com