Conjugated polymer, and preparation method and application thereof
A technology of polymers and groups, applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve problems such as reduced energy conversion efficiency, no increase in device open circuit voltage, and limited application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1, 2,6-bis(trimethyltinyl)-4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)-benzo[1,2-b : Synthesis of 4,5-b']dithiophene
[0066] chemical reaction flow chart Figure 7 Shown, concrete reaction steps and reaction conditions are as follows:
[0067]Compound 1: Under argon protection, lithium diisopropylamide (2.0M, 12mL) was slowly injected into 3-bromo-2-(2-ethylhexyl)thiophene (6.6g, 24mmol) at -78°C ) in tetrahydrofuran (30mL), react at low temperature for 1 hour, then slowly rise to room temperature; then add 4,8-dihydrobenzo[1,2-b:4,5-b']dithiophene-4,8 - Diketone (1.76g, 8.0mmol), heated to 50°C for 2 hours, cooled to room temperature, then added a mixture of stannous chloride dihydrate (12.6g, 56mmol) and dilute hydrochloric acid (10%, 25mL) , react overnight at 50°C. Then the mixture was poured into ice water, extracted several times with diethyl ether, the organic phases were combined, dried with magnesium sulfate, and the dark yellow liquid was obta...
Embodiment 2
[0077] Example 2. Poly{[4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2yl)-benzo[1,2-b:4,5-b']dithiophene -2,6-diyl]-co-[2-hexyldecyl-4,6-bis(thiophen-2-yl)-3-fluoro-thiophene[3,4-b]thiophene-2-ester-5 ,5'-diyl]} (polymer PBT-3F) synthesis.
[0078] chemical reaction flow chart Figure 8 Shown, concrete reaction steps and reaction conditions are as follows:
[0079] Take the monomer 2,6-bis(trimethyltinyl)-4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)-benzo[1,2-b: 4,5-b']dithiophene (compound 5, 0.235 g, 0.25 mmol) and the monomer 2-hexyldecyl-4,6-bis(5-bromothiophen-2-yl)-3-fluoro-thiophene [ 3,4-b] thiophene-2-ester (0.187g, 0.25mmol), dissolved in toluene (10mL), exhausted with argon for 20 minutes, then added the catalyst tetrakis (triphenylphosphine) palladium (0 ) (20mg) and continue to expel the air for 30 minutes. Polymerization was then stopped at reflux temperature of toluene for 12 hours. The polymer solution was cooled to room temperature, slowly poured into me...
Embodiment 3
[0080] Example 3. Processability of the polymers of the invention and measurement of the optical bandgap using absorption spectroscopy
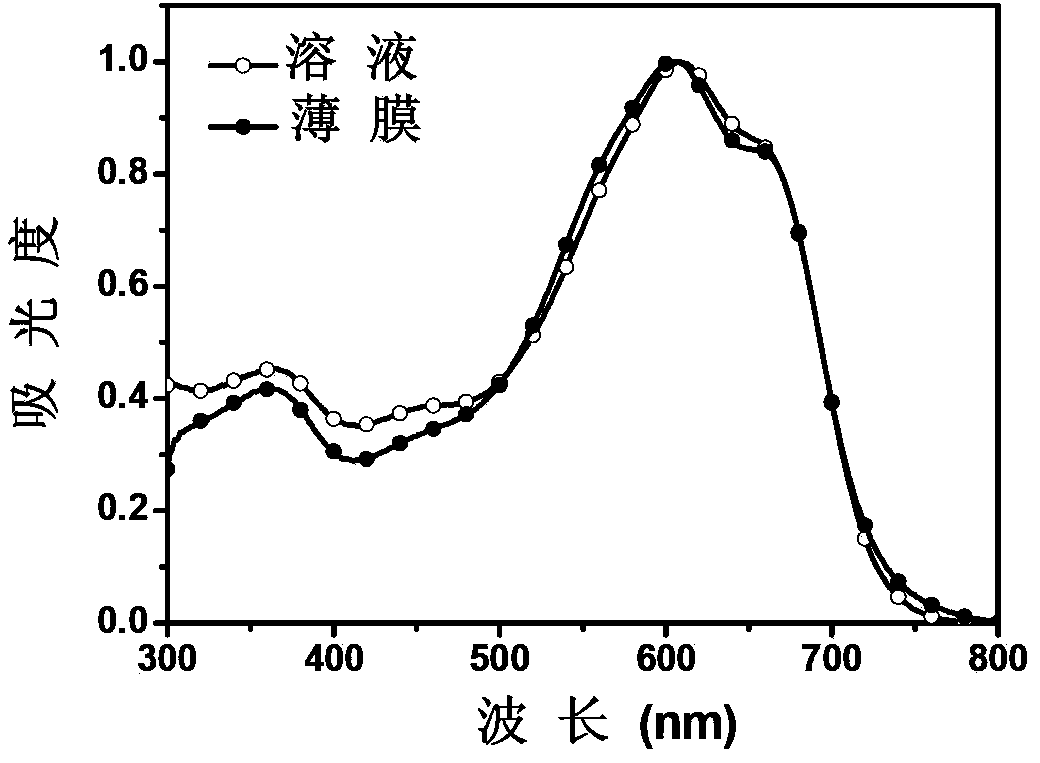
[0081] The polymer prepared in Example 2 was mixed with various organic solvents, including chlorinated solvents such as chloroform, dichloromethane, chlorobenzene and dichlorobenzene, and other solvents such as methanol, toluene and tetrahydrofuran. The polymer PBT-3F was found to have good solubility in chlorinated solvents, but insoluble in methanol. High-quality films were prepared by spin-coating polymer PBT-3F in dichlorobenzene onto glass slides.
[0082] The absorption spectrum that the polymkeric substance that embodiment 2 prepares records under chloroform solution and film state is shown in image 3 . The optical bandgap of polymers uses the empirical formula (Eg=1240 / λ 吸收起始 , where: E g is the optical bandgap of the polymer; λ 吸收起始 is the starting point of the absorption spectrum in the long-wave direction) and is calculated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com