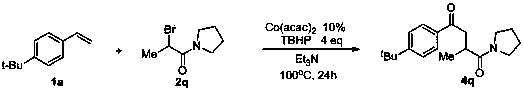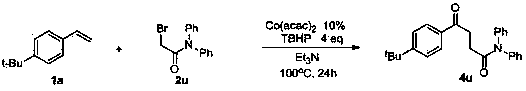Method for preparing 1,4-dicarbonyl derivative
A technology of derivatives and dicarbonyl, applied in the field of preparation of organic compounds, can solve the problems of narrow use range of substrates, narrow range of applicable substrates, harsh reaction conditions, etc., to avoid the use of precious metal catalysts, easy to obtain, and short reaction time short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Cobalt acetylacetonate (Ⅱ) Co(acac) 2 (0.2 mmol, 51.6 mg), compound 1a (2 mmol, 380 uL), compound 2a (6 mmol, 816 uL), tert-butanol hydroperoxide TBHP (1.1 mL), Et 3 N 8 mL was charged into the reactor; then the system was heated in the air at 100 °C for about 24 hours, quenched with saturated sodium sulfite, extracted with ethyl acetate (40 mL × 3), dried over anhydrous sodium sulfate, and passed a simple The product can be obtained by column chromatography 3a , the yield is 86%.
[0024] 1 H NMR (400 MHz, CDCl 3 ) δ 7.92 (d, J = 8.0 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H), 4.15 (q, J = 8.0 Hz, 2H), 3.50 – 3.42 (m, 1H), 3.16 – 3.06 (m, 1H), 3.05 – 2.96 (m, 1H), 1.34 (s, 9H), 1.30 – 1.22 (m, 6H); 13 C NMR (100 MHz, DMSO) δ 197.6, 175.1, 156.2, 133.9, 127.8, 125.4, 59.8, 41.3, 34.8, 34.5, 30.7, 16.9, 14.0. HRMS: Anal. 17 h 24 NaO 3 : 299.1623, Found: 299.1630. IR (KBr, cm -1 ): ν 2970, 2907, 2874, 1732, 1689, 1606, 1568, 1406.
Embodiment 2
[0026]
[0027] Cobalt acetylacetonate (Ⅱ) Co(acac) 2 (0.1 mmol, 25.8 mg), compound 1a (2 mmol, 380 uL), compound 2a (6 mmol, 816 uL), tert-butanol hydroperoxide TBHP (1.1 mL), Et 3 N 8 mL was loaded into the reactor; then the system was heated in the air at 100 °C for about 24 hours, quenched with saturated sodium sulfite, extracted with dichloromethane (40 mL × 3), dried over anhydrous sodium sulfate, and passed a simple The product can be obtained by column chromatography 3a , the yield is 75%.
Embodiment 3
[0029]
[0030] Cobalt acetylacetonate (Ⅱ) Co(acac) 2 (0.4 mmol, 103.2 mg), compound 1a (2 mmol, 380 uL), compound 2a (6 mmol, 816 uL), tert-butanol hydroperoxide TBHP (1.1 mL), Et 3 N 8 mL was charged into the reactor; then the system was heated in the air at 100 °C for about 24 hours, quenched with saturated sodium sulfite, extracted with ethyl acetate (40 mL × 3), dried over anhydrous sodium sulfate, and passed a simple The product can be obtained by column chromatography 3a , the yield is 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com