A kind of synthesis method of nanometer magnesium fluoride of high specific surface area
A synthesis method, magnesium fluoride technology, applied in nanotechnology for materials and surface science, magnesium fluoride, magnesium halide, etc., can solve the problem of low yield of magnesium fluoride on high specific surface, harsh production or reaction conditions, difficult Realize industrialization and other issues, achieve the effect of reducing economic waste and environmental pollution, suitable for promotion and application, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The production method of high specific surface area nano-magnesium fluoride in the present embodiment, comprises the following steps:
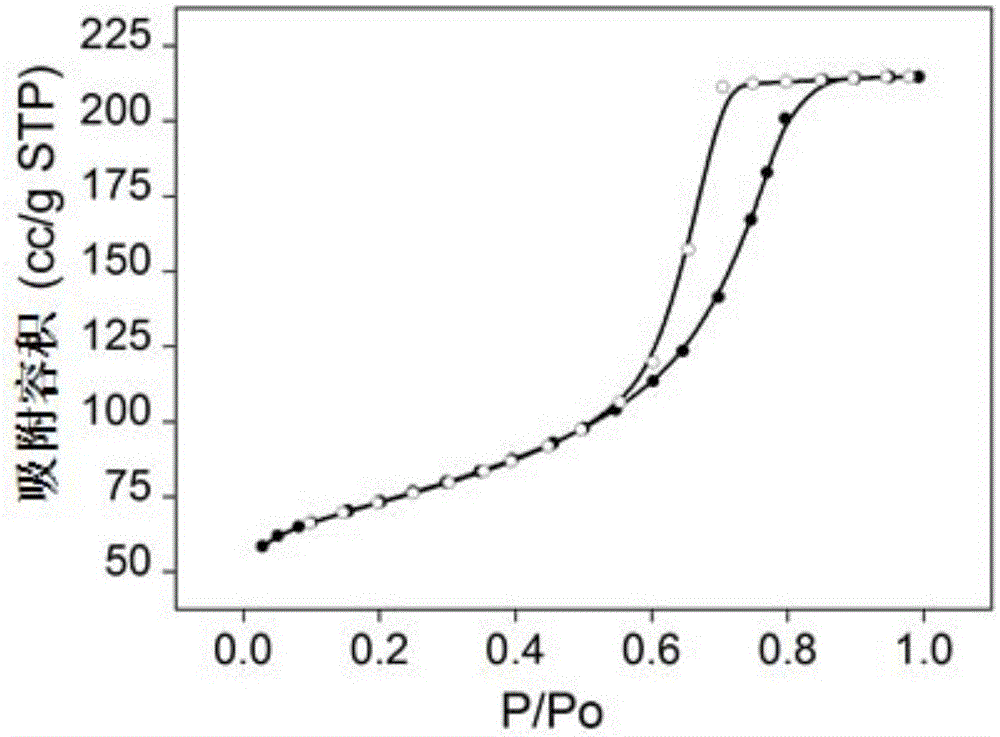
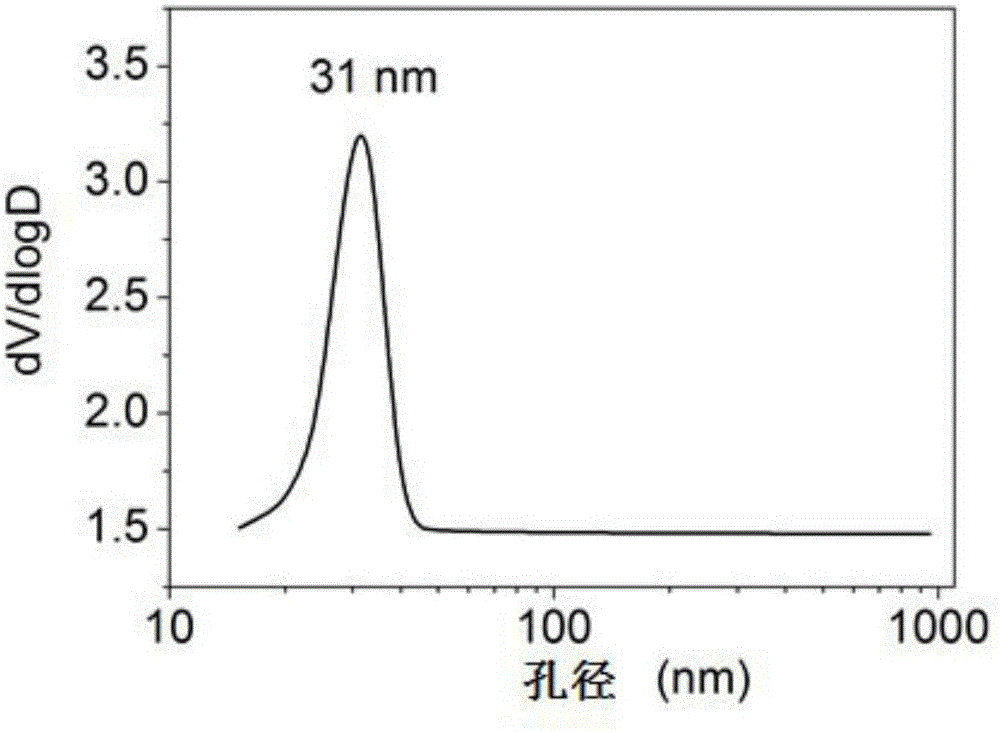
[0037] Prepare an equal volume of 3mol / L NH 4 F solution and 1mol / L Mg(CH 3 COO) 2 4H 2O solution, mixed by positive addition, and at the same time turned on the magnetic stirring, reacted at a temperature of 30 ° C for 7 hours, and then stood at a constant temperature for aging for 2 hours, suction filtered the obtained colloidal solution, took its filter cake, and placed it in After drying for 4 hours in the air flow at ℃, put it in an atmospheric oven at 110℃ and dry it for 17 hours, and finally bake it in a muffle furnace under an air atmosphere at 200℃ for 4 hours to obtain a transparent magnesium fluoride product. 153m 2 / g, the pore volume is 0.29cc / g, and the most probable pore size distribution is 31nm.
[0038] The high specific surface area magnesium fluoride nitrogen adsorption isotherm figure, pore size distribution fi...
Embodiment 2
[0040] The production method of high specific surface area nano-magnesium fluoride in the present embodiment, comprises the following steps:
[0041] Prepare an equal volume of 2mol / L NH 4 F solution and 1mol / L Mg(CH 3 COO) 2 4H 2 O solution, reacted by co-current co-precipitation method, and at the same time turned on the magnetic stirring, reacted at a temperature of 50 ° C for 5 hours, and then stood at constant temperature for aging for 1 hour, and suction-filtered the obtained solid-liquid mixture. After drying the cake in an air stream at 25°C for 6 hours, put it in an oven at 100°C and dry it for 20 hours, and finally bake it in a muffle furnace under a nitrogen atmosphere at 300°C for 3 hours to obtain magnesium fluoride powder. The specific surface area is 146m 2 / g, the pore volume is 0.31cc / g, and the most probable pore size distribution is 36nm. The XRD pattern of gained magnesium fluoride and image 3 similar.
Embodiment 3
[0043] The production method of high specific surface area nano-magnesium fluoride in the present embodiment, comprises the following steps:
[0044] Prepare an equal volume of 4mol / L NH 4 F solution and 2mol / L Mg(CH 3 COO) 2 4H 2 O solution, mixed by reverse addition, and at the same time turned on the magnetic stirring, reacted at a temperature of 70 ° C for 12 hours, and then stood at a constant temperature for aging for 3 hours, and suction-filtered the obtained colloidal solution, and took its filter cake. After drying in the air flow at ℃ for 1 hour, put it in an oven at 90 ℃ and dry it for 25 hours, and finally bake it in a muffle furnace under a nitrogen atmosphere at 500 ℃ for 2 hours to obtain a transparent magnesium fluoride product with a specific surface area of 139m 2 / g, the pore volume is 0.31cc / g, and the most probable pore size distribution is 34nm. The XRD pattern of gained magnesium fluoride and image 3 similar.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com