2-fuloro fumarate (formula I), and preparation method and application thereof
The technology of dialkyl fluorofumarate and dialkyl fumarate is applied in the field of dialkyl 2-fluorofumarate and its preparation, and can solve the immunosuppressive effect of fluorofumarate Unmanned research and other problems, to achieve the effect of increasing the difficulty of purification, easy control, and reducing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
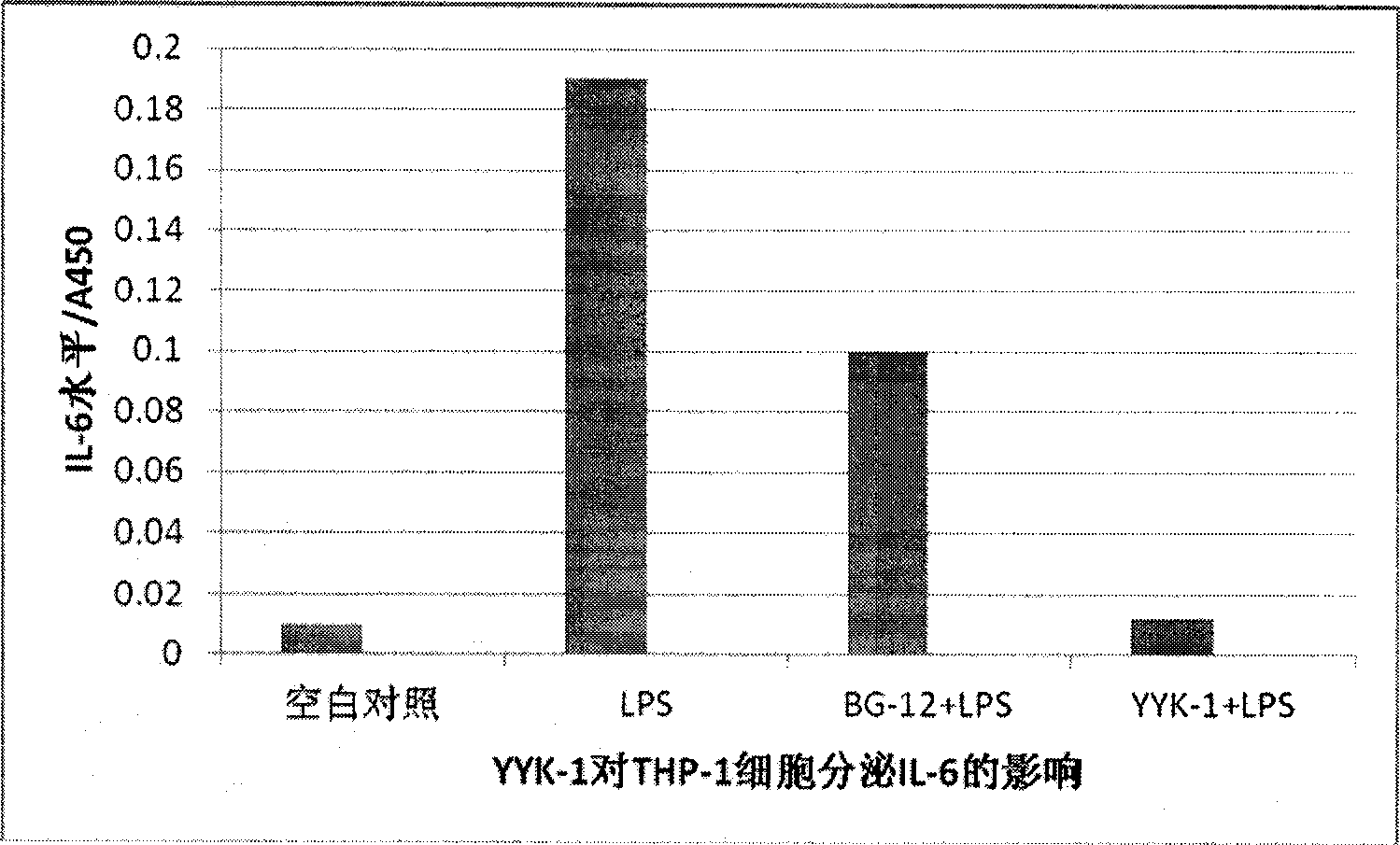
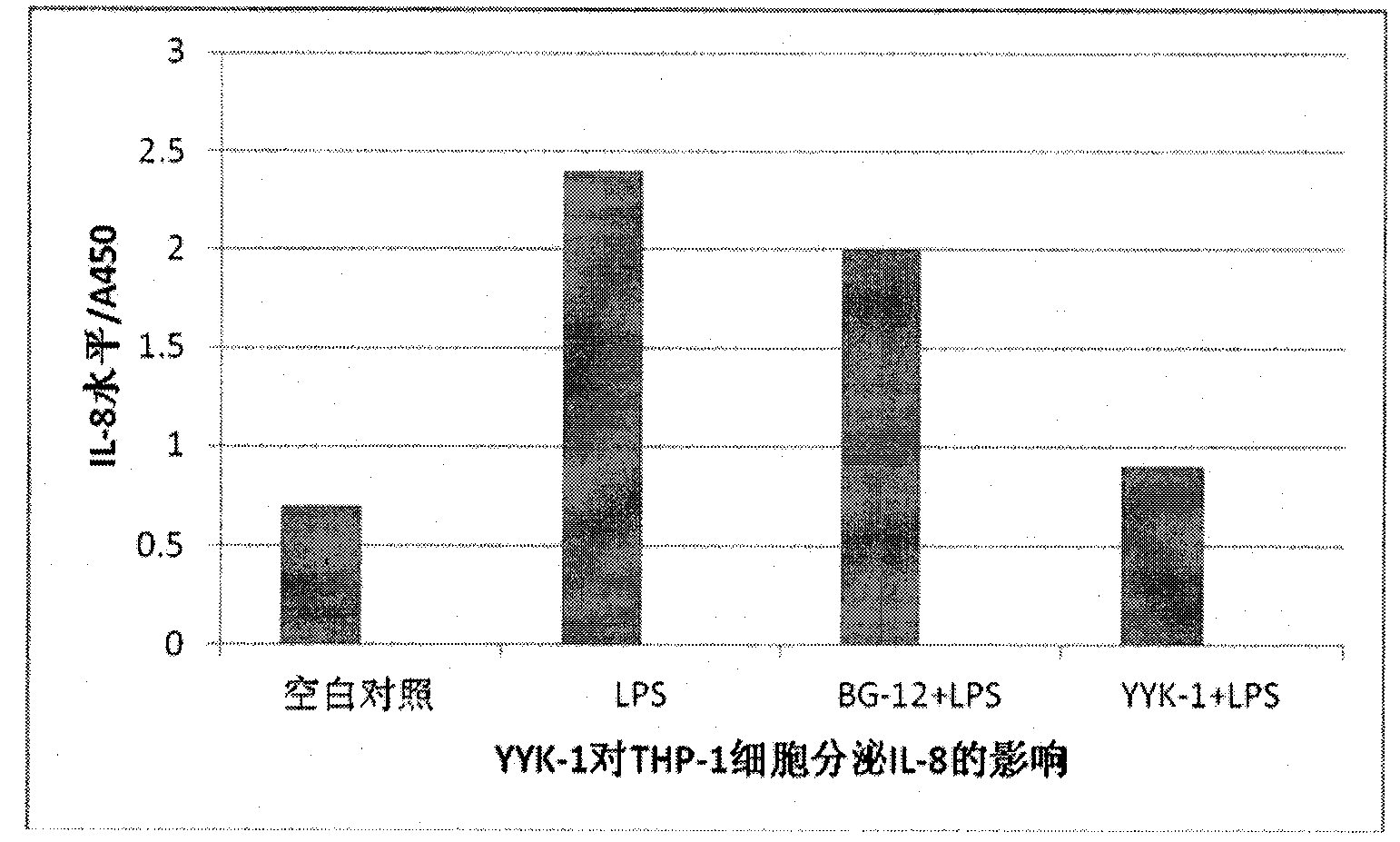
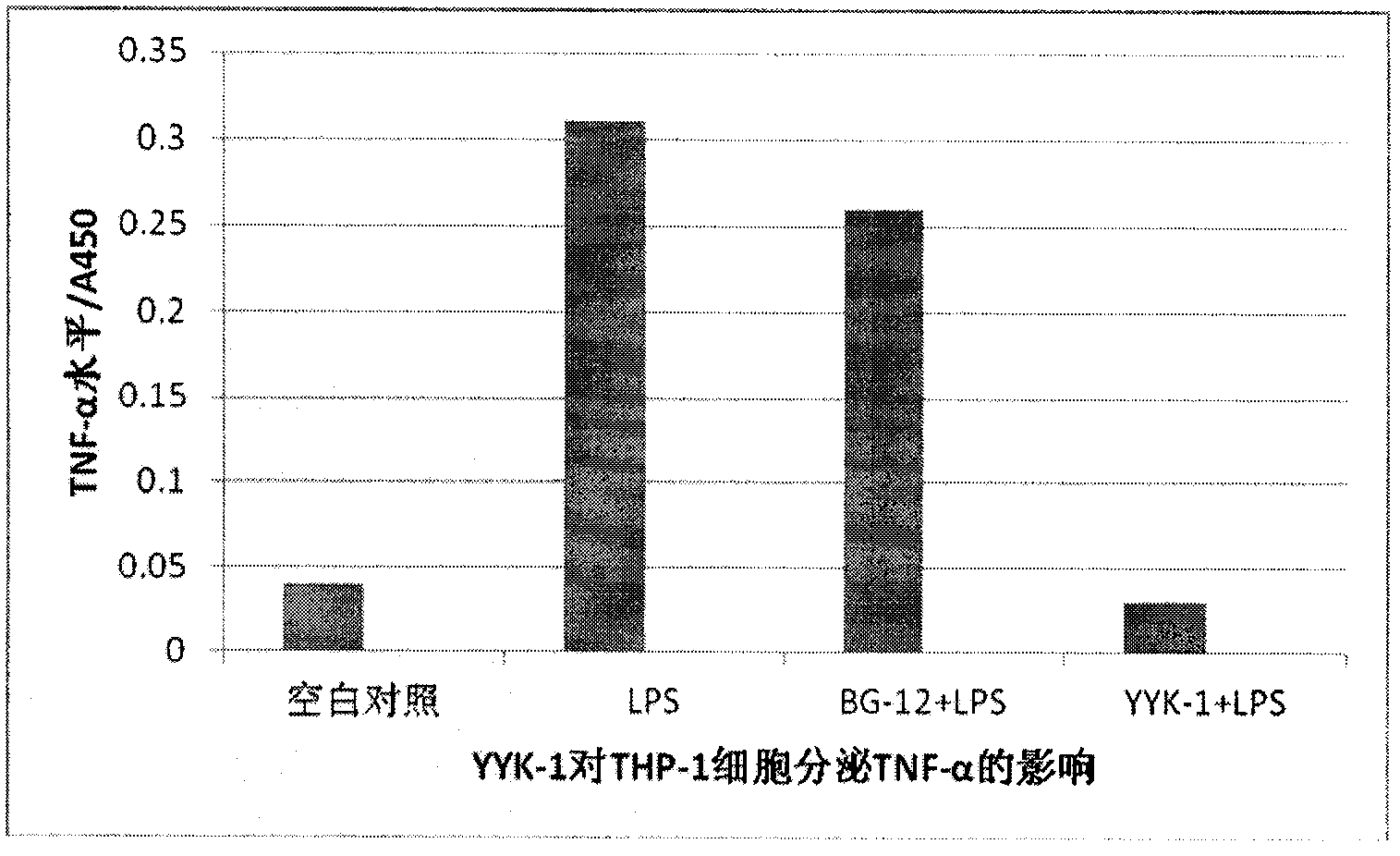
[0044] Preparation and biological activity of diethyl 2-fluorofumarate (compound YYK-1)
[0045] 1. Add ethanol (1.2g, 26mmol), butynedioic acid (1g, 8.77mmol), p-toluenesulfonic acid (30mg, 0.17mmol) and 1,2-dichloroethane (50mL) into a 250mL round bottom in the flask. After refluxing for 12 hours, add 50 mL of toluene and continue refluxing to separate water (using a water separator) for 8 hours. Concentrate and purify by flash chromatography (eluent PE / EA=98 / 2) to obtain diethyl butynedioate (1.35 g, yield 91%).
[0046] 2. Add diethyl butynedioate (1.1g, 6.46mmol), potassium fluoride (0.73g, 12.6mmol), DMF (50mL), acetonitrile (50mL) into a round bottom flask, add water (0.48mL) under stirring mL, 26.7 mmol) and stirred at 80°C for 2 hours. Cool to room temperature, add ethyl acetate (100mL) and transfer the solution into a separatory funnel, wash with saturated brine (4×30mL), dry the organic layer over anhydrous sodium sulfate, concentrate to give a brown liquid, pass...
Embodiment 2
[0058] Preparation of Dimethyl 2-Fluorofumarate (Compound YYK-2)
[0059] Add methanol, butynedioic acid, p-toluenesulfonic acid and 1,2-dichloroethane into a 250 mL round bottom flask. After refluxing for 8-12 hours, add toluene and continue refluxing to separate water (using a water separator) for 6-8 hours. Concentrate, and purify by flash chromatography (eluent PE / EA) to obtain dimethyl butyndioate (93% yield).
[0060] Add dimethyl butyndioate, potassium fluoride, DMF, and acetonitrile into a round bottom flask, add water while stirring and stir at 80°C for 2-3 hours. Cool to room temperature, add ethyl acetate and transfer the solution into a separatory funnel, wash with saturated brine, dry the organic layer over anhydrous sodium sulfate, and concentrate to obtain a brown liquid, which is subjected to silica gel column chromatography (eluent: PE / EA) was purified to obtain dimethyl 2-fluorofumarate (HPLC purity 98.56%, yield 65%).
[0061] 1 H NMR (400M, CDCl 3 ) δ...
Embodiment 3
[0065] Preparation of bis-(2'-ethoxyethyl)-2-fluorofumarate (compound YYK-3)
[0066] Add 2-ethoxyethanol, butynedioic acid, p-toluenesulfonic acid and 1,2-dichloroethane into a 250 mL round bottom flask. After refluxing for 8-16 hours, add toluene and continue refluxing to separate water (using a water separator) for 6-10 hours. Concentrate and purify by flash chromatography (eluent PE / EA) to obtain bis-(2'-ethoxyethyl) butyndioate (yield 78%).
[0067] Add bis-(2'-ethoxyethyl)butyndioate, potassium fluoride, DMF, and acetonitrile into a round bottom flask, add water under stirring and stir at 80°C for 2-3 hours. Cool to room temperature, add ethyl acetate and transfer the solution into a separatory funnel, wash with saturated brine, dry the organic layer over anhydrous sodium sulfate, and concentrate to obtain a brown liquid, which is subjected to silica gel column chromatography (eluent: PE / EA) was purified to obtain bis-(2'-ethoxyethyl)-2-fluorofumarate (yield 47%).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com