Method for synthesizing alliin with optical activity and reaction device thereof
A synthetic method and optically active technology, applied in chemical instruments and methods, organic chemistry, preparation of organic compounds, etc., can solve the problems of shortage of bromine resources, difficult separation, high reactivity, etc., to improve body immunity, promote blood The effect of circulation and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
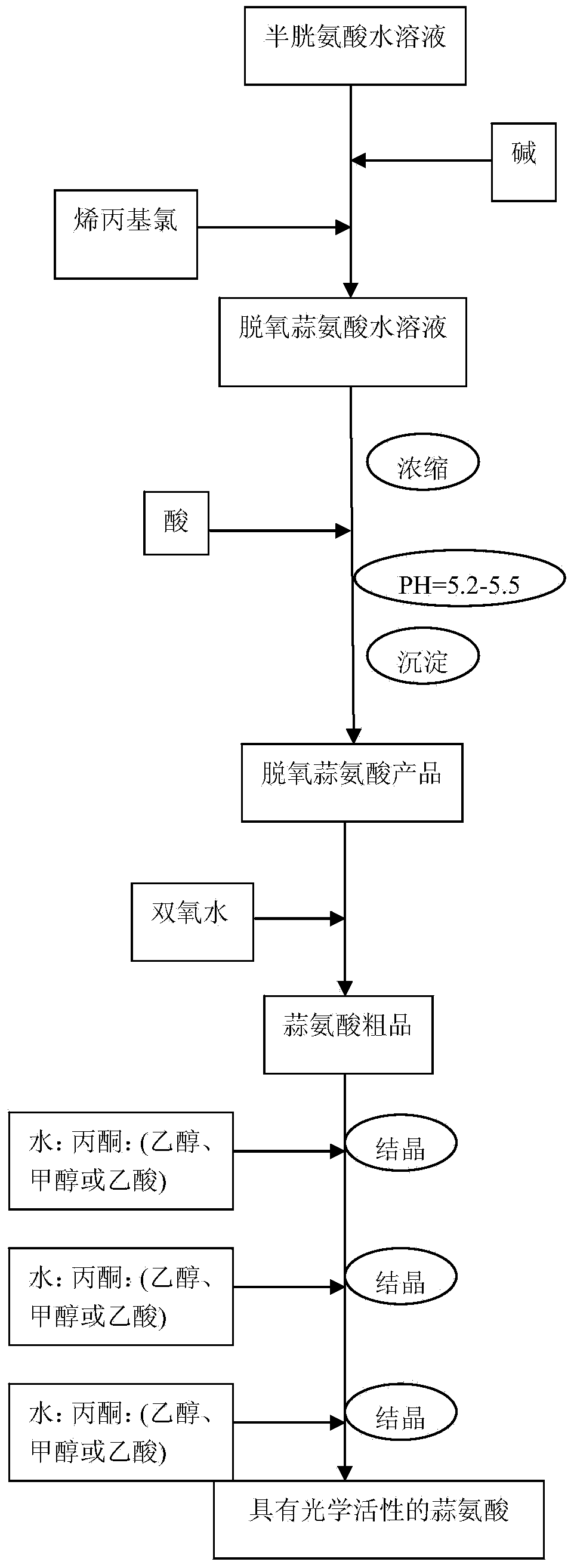
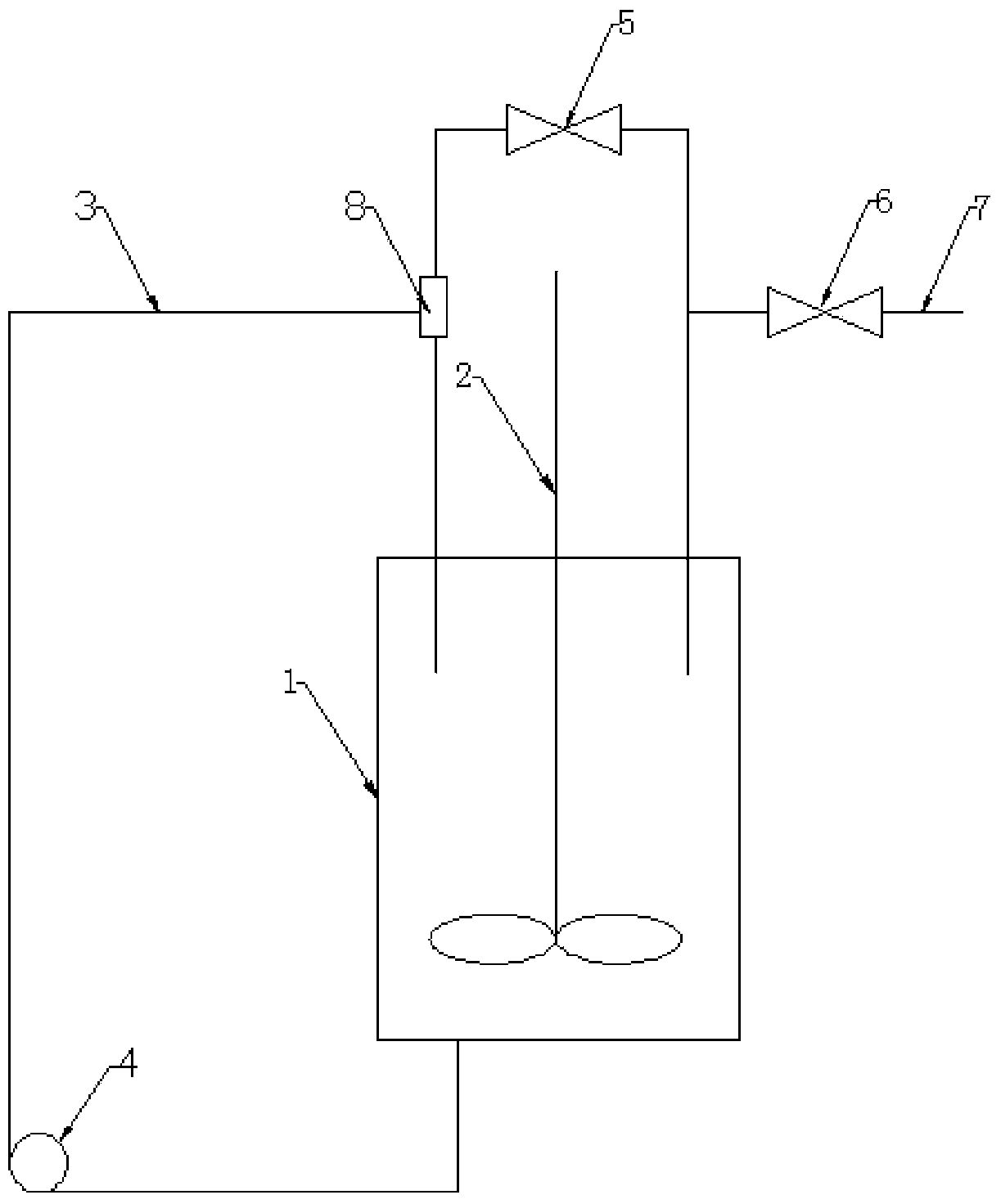
[0035]Step 1, first cool the closed reactor 1 with an ice bath until the temperature is 0°C, open the feed valve 6, close the circulation valve 5, and feed nitrogen into the closed reactor 1 from the feed pipe 7 at a flow rate of 1.0 L / min , then add 100 milliliters of 1mol / L cysteine aqueous solution (containing 12.1 grams of cysteine) to it, close the feed valve 6, then open the circulation valve 5, and stir for 5 minutes with the stirring paddle 2 at a speed of 500 rpm; when the temperature of closed reactor 1 is controlled within 10°C, add 110 ml of 1mol / L NaOH solution and continue stirring (500 rpm) until all cysteine is dissolved, then add 0.12 mol of allyl chloride (about 9.2 grams), reacted at this temperature for 2.5 hours to obtain a deoxyalliin aqueous solution, and then stirred at room temperature for 1 hour at a speed of 500 rpm; then extracted three times with ethyl acetate (each 200mL each time), the extracted aqueous solution was vacuum concentrated at 60°...
Embodiment 2
[0040] Step 1, when cooling the reactor with an ice bath until the temperature is 5°C, feed nitrogen into the reactor with a flow rate of 0.5L / min, and then add 50 ml of 1mol / L cysteine aqueous solution (containing cysteine amino acid 6.0 g), and turn on the stirrer for 3 minutes, the stirring speed is 400 rpm, and the temperature of the reactor is controlled within 10°C, then add 55 ml of 1mol / L NaOH solution, and continue to stir (400 rpm / min) until the cysteine is completely dissolved, then add 0.06mol of allyl chloride, react at this temperature for 2.5 hours to obtain a deoxyalliin aqueous solution, and then stir for 1 hour at room temperature, with a speed of 400 rpm ; Then extract three times with ethyl acetate (200mL each time), concentrate the extracted aqueous solution to one-third of the original volume by vacuum at 60°C, and adjust the pH value to 5.5 with hydrochloric acid after the concentrated solution is cooled to room temperature. Stand still for 12 hours...
Embodiment 3
[0044] Step 1, when cooling the reactor with an ice bath until the temperature is 5°C, feed nitrogen into the reactor with a flow rate of 10 L / min, and then add 10 milliliters of 1mol / L cysteine aqueous solution (containing cysteine acid 1.2 g), stirred for 3 minutes, the rotation speed was 500 rpm, and when the temperature of the reactor was controlled within 10°C, 0.8 ml of 17mol / L ammonia solution was added, and the stirring was continued until the cysteine was completely dissolved. The number of rotations is 500 rpm, then add 0.01mol of allyl chloride (1.0 g), and react at this temperature for 2.0 hours to obtain a deoxyalliin aqueous solution, then stir at room temperature for 1 hour, and the number of rotations is 500 rpm ; Then extract three times with petroleum ether (10 mL each time), concentrate the extracted aqueous solution to one-third of the volume of the original solution in vacuum at 80 ° C, and adjust the pH value to 5.5 with hydrochloric acid after the con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com