Synthesis method of lasofoxifene precursor of nafoxidine
A technology of nafaxidine and its synthesis method, which is applied in the field of chemical synthesis, can solve the problems of different process conditions, reaction temperature and separation method, etc., and achieve the effects of mild reaction conditions, easy control of reaction conditions, and solution to source problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 6-methoxy-2-phenyl-1-tetralone ( 2 ) (15.0 g, 59.5 mmol) was dissolved in dichloromethane (120 mL), and DBU (12.6 g, 83.3 mmol) was added. Perfluorobutylsulfonyl fluoride (25.2 g, 83.3 mmol) was slowly added to the reaction solution, and the addition was completed in about 20 minutes. Stir at room temperature for 16h. Add 120mL of distilled water × 2 to the reaction liquid to wash, and add 167mL of 0.5N hydrochloric acid solution, separate the water phase, and extract with 100mL of dichloromethane × 2, combine the organic phases, wash with 100mL of saturated sodium chloride solution, and dry over anhydrous sodium sulfate , filter, and concentrate the filtrate to obtain 6-methoxy-2-phenyl-3,4-dihydronaphthalene-1-perfluorobutanesulfonate ( 3 ) 30.0g, pale yellow oil, yield 94.3%.
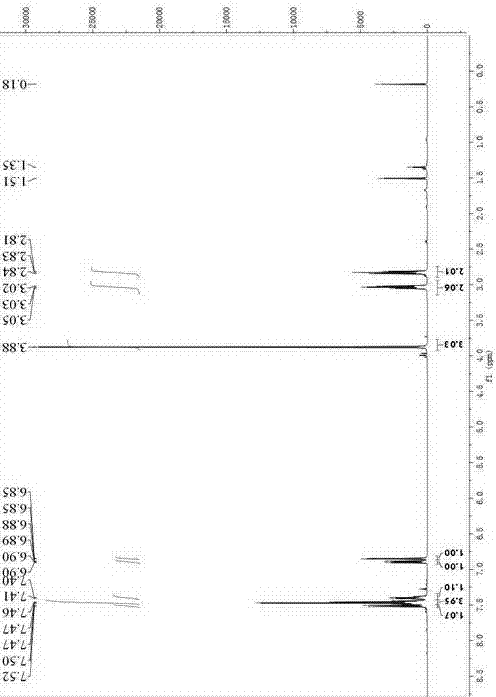
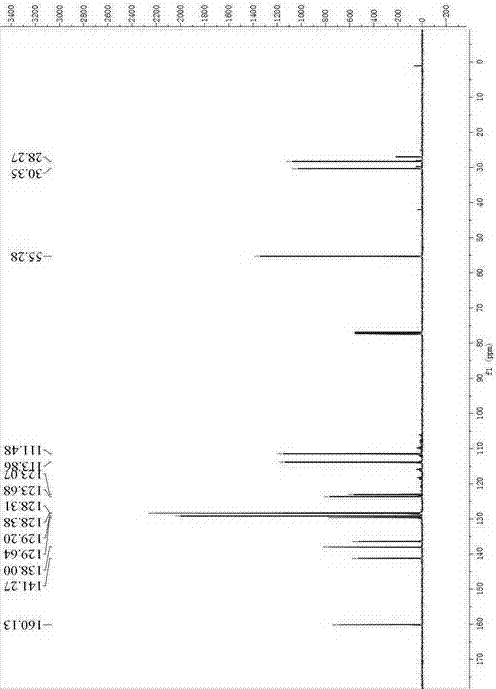
[0042] 6-methoxy-2-phenyl-3,4-dihydronaphthalene-1-perfluorobutanesulfonate ( 3 )of 1 H NMR (500 MHz, CDCl 3 )See figure 1 : δ 7.51 (d, J = 8.5 Hz, 1H), 7.49 – 7.43 (m, 4H), 7.43 – ...
Embodiment 2
[0045] 6-methoxy-2-phenyl-1-tetralone ( 2 ) (3.00 g, 11.9 mmol) was dissolved in THF (25 mL), and DBU (2.53 g, 16.7 mmol) was added. Perfluorobutylsulfonyl fluoride (5.03 g, 16.7 mmol) was slowly added to the reaction solution, and the addition was completed in about 15 minutes. Stir at room temperature for 16h. THF was evaporated under reduced pressure, 30 mL of dichloromethane was added to the residue, washed with 30 mL of distilled water × 2, and 33.3 mL of 0.5N hydrochloric acid solution was added, the aqueous phase was separated, extracted with 10 mL of dichloromethane, the organic phases were combined, saturated chlorinated Wash with 30 mL of sodium solution, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to obtain 6-methoxy-2-phenyl-3,4-dihydronaphthalene-1-perfluorobutylsulfonate ( 3 ) 5.89g, light yellow oil, yield 92.7%.
Embodiment 3
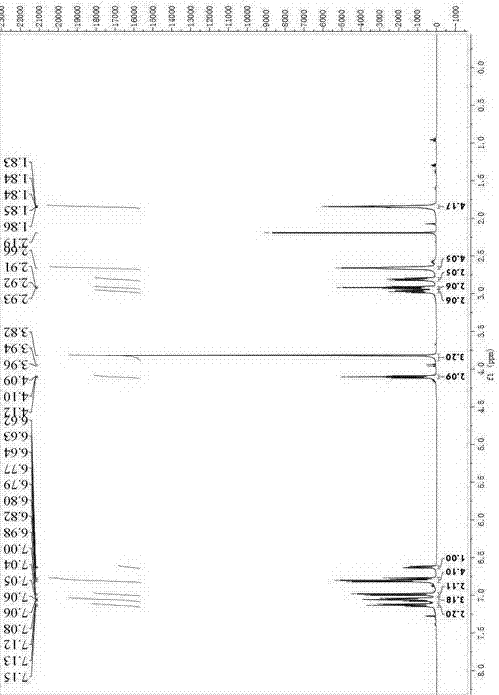
[0047] At 0°C, to (4-(2-(1-pyrrolidinyl)ethoxy)phenylmagnesium bromide ( 4 ) (M = MgBr) (11.2mmol) in THF solution (48mL), add CuCl (1.11g, 11.2mmol), anhydrous LiCl (0.940g, 22.5mmol), stir for 1h, add 6-methoxy- 2-Phenyl-3,4-dihydronaphthalene-1-perfluorobutanesulfonate ( 3 ) (6.00g, 11.2mmol) and iron acetylacetonate (3.97g, 11.2mmol), stirring was continued at 0°C for 2h. The reaction solution was poured into saturated ammonium chloride solution (50 mL), the solid was removed by filtration, and the filtrate was collected. The aqueous phase was separated and extracted with 20 mL of ethyl acetate × 2, the organic phases were combined, and the solvent was evaporated under reduced pressure. Add 40 mL of distilled water and 2N hydrochloric acid to the residue to make the pH 2~3, add 20 mL of a mixture of diethyl ether and methyl tetrahydrofuran (1:3 v / v) for extraction, separate the organic phase, and use 0.5 N Extract with 30mL of hydrochloric acid, combine the water phases...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com