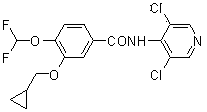
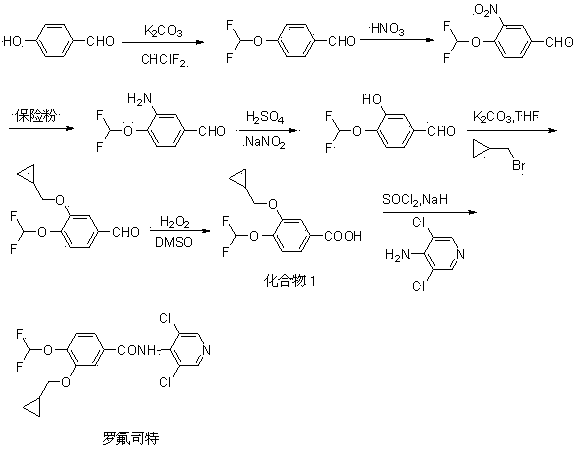
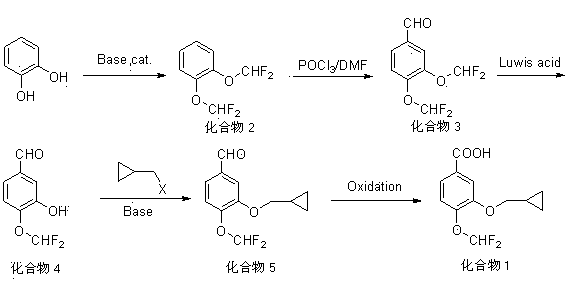
Method for synthesizing roflumilast intermediate
A synthetic method, the technology of roflumilast, applied in the field of medicine and chemical industry, can solve the problems of harsh process conditions, low yield, high cost of raw materials, etc., and achieve the effect of cheap and easy-to-obtain raw materials, simple process principle, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 1,2-bis(difluoromethoxy)benzene (compound 2):
[0048] Add 11.0 g (100 mmol) of catechol and 110 ml of DMF to the reaction flask, add 5.1 g (210 mmol) of sodium hydride in batches under stirring, raise the temperature to 80°C, and pass through chlorodifluoromethane to keep the reaction for 8 h. TLC monitored the complete conversion of the reactant to the product (developing solvent: ethyl acetate: n-hexane = 1:3). After the reaction was completed, the solvent DMF was recovered under reduced pressure. After cooling to room temperature, about 200 mL of water was added, and 80 ml of dichloromethane was added to extract three times. Dry over magnesium sulfate, filter out the desiccant, and concentrate under reduced pressure until there is no slip-out product to obtain 17.2 g of oily substance, with a yield of 82%, which is directly used in the next reaction without purification. ESI-MS (m / z ) : 211 [M+1] + .
[0049] Synthesis of 3,4-bis(difluoromethoxy)benz...
Embodiment 2
[0059] Synthesis of 1,2-bis(difluoromethoxy)benzene (compound 2)
[0060] Add 11.0 g (100 mmol) of catechol and 80 ml of DMF to the reaction flask, add 11.4 g (220 mmol) of sodium ethoxide in batches under stirring, heat to 80°C, pass through chlorodifluoromethane, and react for 12 h. TLC monitored the complete conversion of the reactant to the product (developing solvent: ethyl acetate: n-hexane = 1:3). Most of the DMF was recovered by distillation. After cooling to room temperature, add about 200 mL of water, add 80 ml of dichloromethane to extract three times, combine the organic layers, wash with 100 ml of 5% NaOH, wash twice with 100 ml of water until neutral, and dry with 18 g of anhydrous magnesium sulfate. Filter off the desiccant, concentrate under reduced pressure until there is no slip-out, and obtain 16.8 g of oil, with a yield of 80%, which is directly used in the next reaction without purification.
[0061] Synthesis of 3,4-bis(difluoromethoxy)benzaldehyde (comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com