Compound antibacterial dry suspension for livestock and poultry and preparation method thereof
An antibacterial dry and suspension technology, applied in the directions of antibacterial drugs, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of inconvenient clinical administration, complex preparation process, poor compliance, etc., and achieve drug availability. High, simple process, improved utilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The preparation of embodiment 1 dry suspension of the present invention
[0017] Raw material prescription:
[0018]
[0019] Process:
[0020] (1) Weigh the sucrose, crush it, pass through an 80-mesh sieve, and set aside.
[0021] (2) Weigh the prescribed amount of sulfamethoxine, enrofloxacin, sucrose powder, sodium carboxymethylcellulose (CMC-NA), xanthan gum, and sodium citrate, dry them separately and mix them evenly. Mesh sieve, spare.
[0022] (3) Take the mixed powder prepared in (2), make a soft material with 60% ethanol and granulate it with an 18-mesh sieve, dry it in an oven at 60°C, add the prescribed amount of milk essence, and pack 100g after whole grain , that is.
Embodiment 2
[0023] The screening test of embodiment 2 drug prescription of the present invention
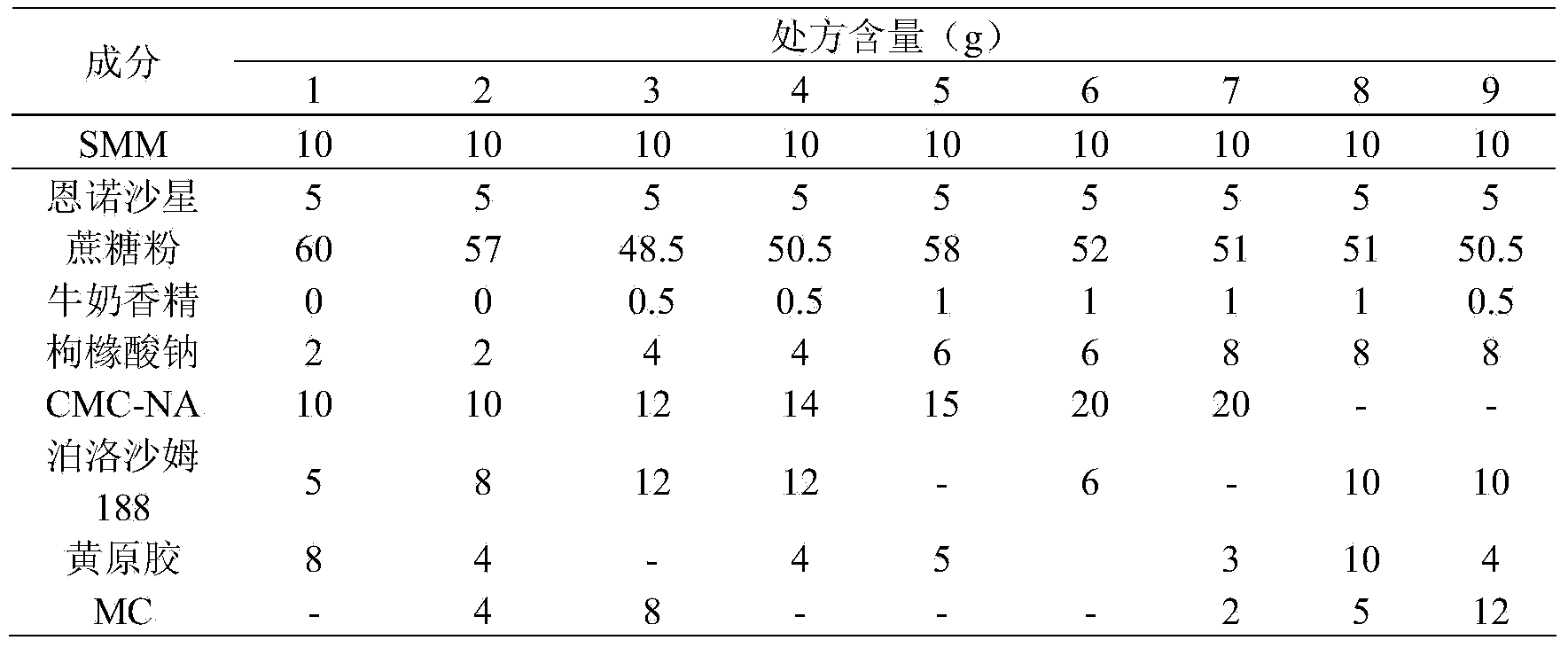
[0024] Table 1 Dry Suspension Prescription Screening Form
[0025]
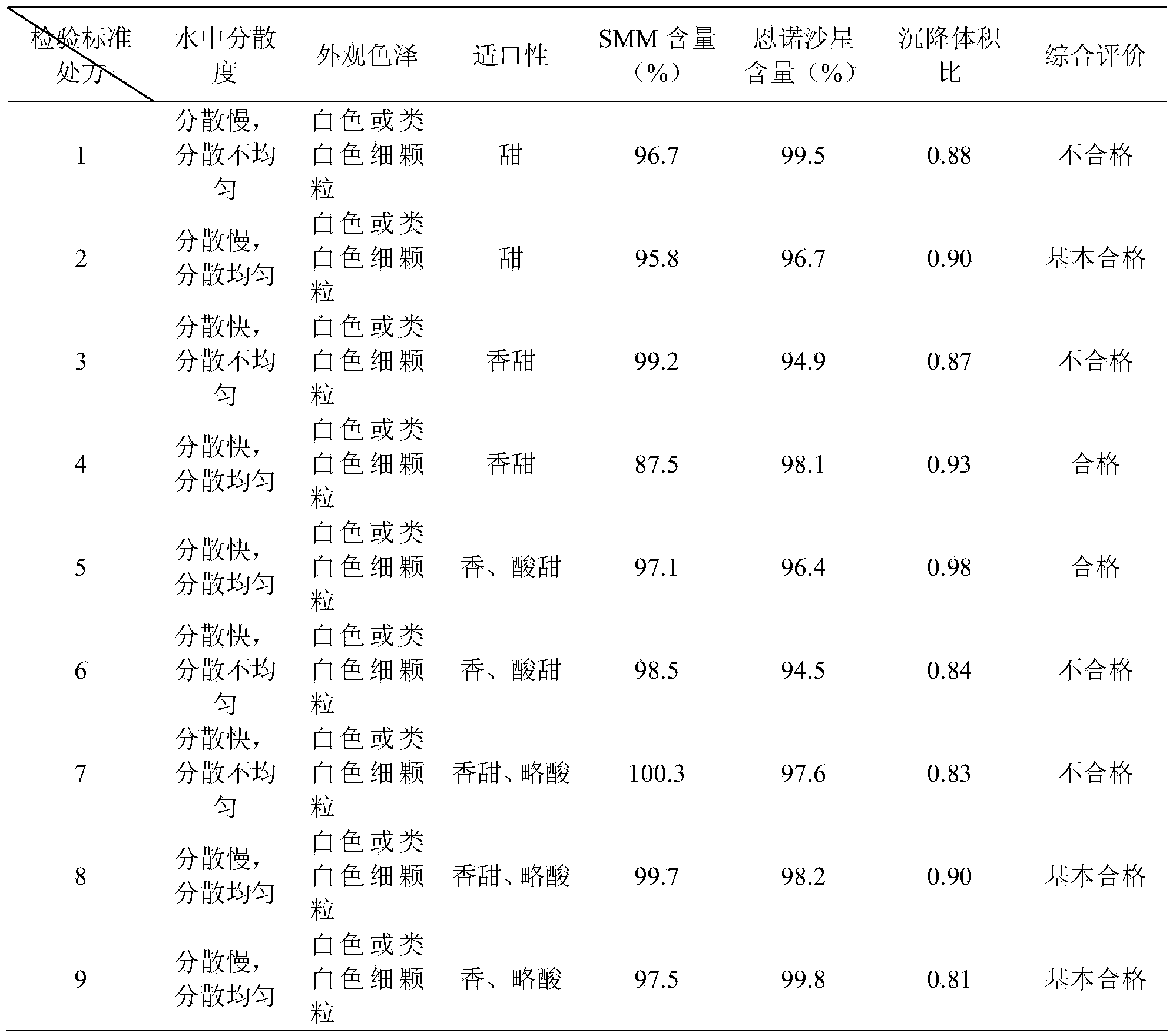
[0026] Table 2 Prescription Screening Results
[0027]
[0028] As can be seen from the above Table 1 and Table 2, the present invention finally obtains a qualified dry suspension with good dispersibility, high sedimentation volume ratio and good mouthfeel through the examination of the consumption of raw and auxiliary materials, wherein the comprehensive evaluation of prescriptions 4 to 5 is qualified, and Prescription 5 is the best. The final selected prescription 5 is the final prescription.
Embodiment 3
[0029] Embodiment 3 dry suspension stability detection test of the present invention
[0030] Three batches of dry suspension were prepared according to the optimal prescription and placed under the conditions of 40°C and 75% humidity for 6 months, and their content, sedimentation volume ratio, redispersibility, moisture content and other indicators were measured. The final results are shown in Table 3.
[0031] Table 3 The compound dry suspension was placed for 6 months at 40°C and 75% humidity
[0032]
[0033] The results showed that the content of the compound dry suspension particles did not change significantly, and all the detection indexes met the regulations, and the stability was good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com