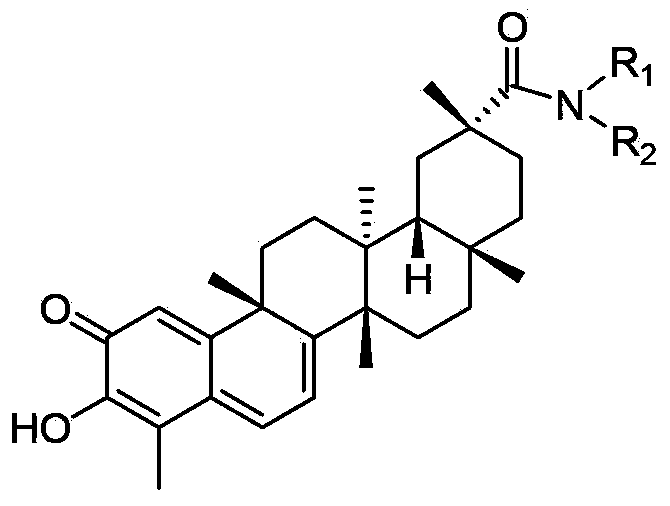
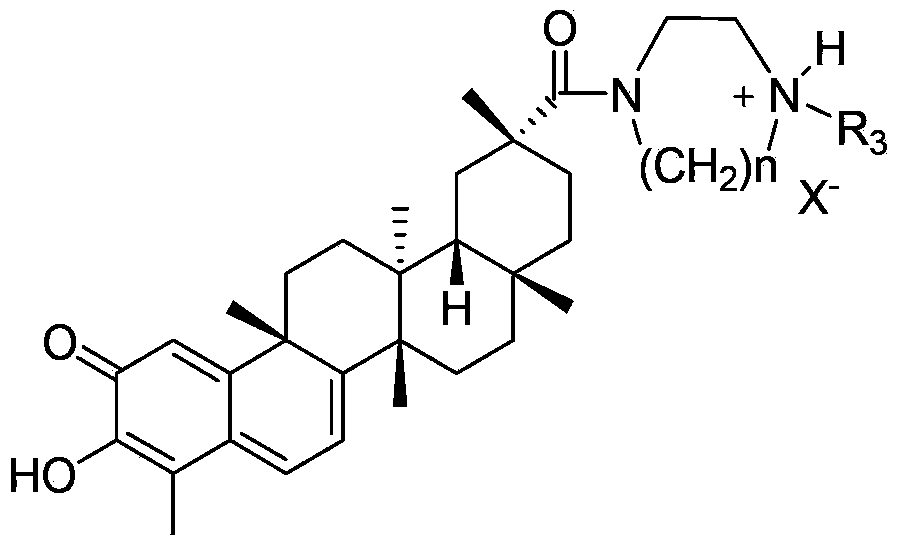
Tripterine derivative, biogenetic salt of derivative, and preparation method and application of biogenetic salt
A technology of tripterine and its derivatives, which is applied in the direction of drug combination, steroids, digestive system, etc., can solve the problems of little research on liver fibrosis, achieve good hepatic stellate cell activation and increase, and improve bioavailability , The effect of water solubility improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
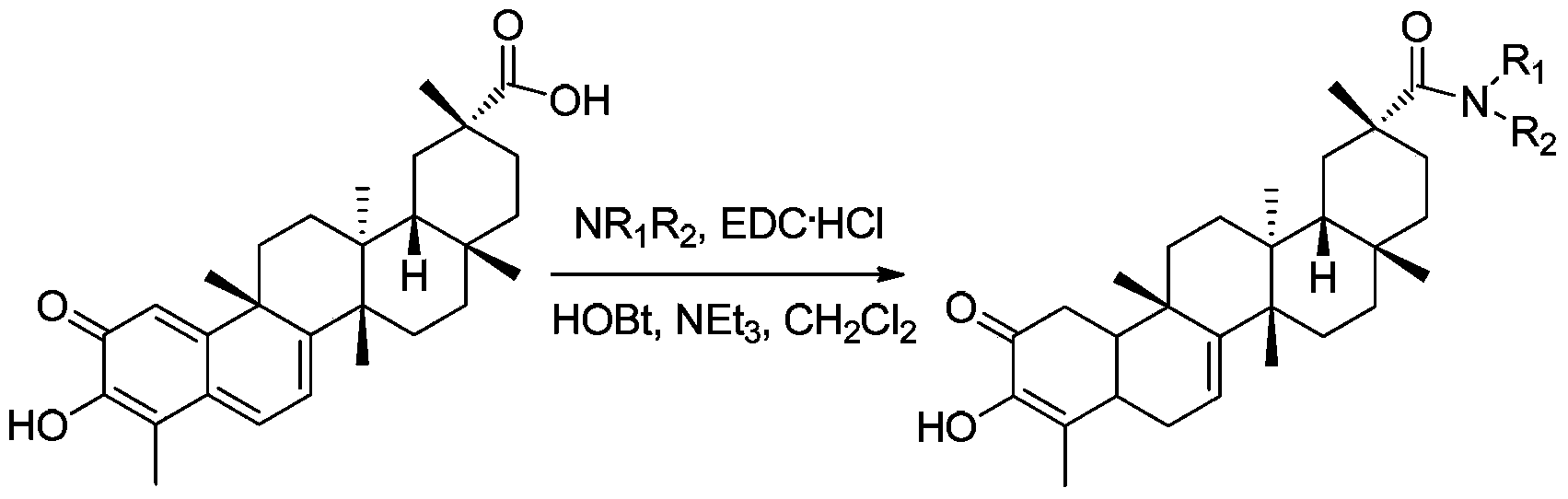
[0030] The preparation of derivative a (synthetic route such as figure 1 shown)
[0031] Dissolve tripterine (20mg, 0.044mmol) in dichloromethane (3ml), add EDC·HCl (43mg, 0.22mmol), HOBT (30mg, 0.22mmol), methylamine hydrochloride (23mg, 0.35mmol) , stirred in an ice bath for 30 min, added triethylamine (50 μl), stirred at room temperature for 12 h, extracted 3 times with dichloromethane, combined organic layers, and anhydrous Na 2 SO 4 Dry and concentrate by rotary evaporation to give the crude product as a dark red oil. The crude product was separated and purified by fast silica gel column chromatography (the mobile phase was a mixture of petroleum ether and ethyl acetate with a volume ratio of 1:1), and the product was dried in vacuo to obtain 8 mg of a dark red solid, which was derived from tripterine. Material a, the yield is 39%.
[0032] The spectral analysis of the tripterine derivative a is as follows: M.p.133°C; 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):6.98(d,1H,J=4.4...
Embodiment 2
[0034] The preparation of derivative b (synthetic route such as figure 1 shown)
[0035] Tripterine (20mg, 0.044mmol) was dissolved in dichloromethane (3ml), added EDC·HCl (43mg, 0.22mmol), HOBT (30mg, 0.22mmol), dimethylamine hydrochloride (30mg, 0.37mmol), ice Stir in the bath for 30 min, add triethylamine (50 μl), stir at room temperature overnight, extract 3 times with dichloromethane, combine organic layers, anhydrous Na 2 SO 4 Dry and concentrate by rotary evaporation to obtain a crude product of dark red oil; the crude product was separated and purified by flash silica gel column chromatography (the mobile phase was a mixture of petroleum ether and ethyl acetate with a volume ratio of 1:1), and dried in vacuo 5 mg of red solid was obtained, which was tripterine derivative b, and the yield was 23%.
[0036] The spectral analysis of the tripterine derivative b is as follows: M.p.142°C; 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):7.04(d,1H,J=6.8Hz,H-6),6.54(s,1H,H-1),6.36(d,1H,J...
Embodiment 3
[0038] The preparation of derivative c (synthetic route such as figure 1 shown)
[0039] Dissolve tripterine (46mg, 0.10mmol) in dichloromethane (3ml), add EDC·HCl (24mg, 0.13mmol), HOBT (17mg, 0.13mmol), ethanolamine (7.9μl, 0.13mmol), stir in ice bath After reacting for 30 min, triethylamine (22 μl) was added, stirred overnight at room temperature, extracted 3 times with dichloromethane, combined organic layers, anhydrous Na 2 SO 4 Dry and concentrate by rotary evaporation to obtain a crude product of a dark red oil; the crude product was separated and purified by flash silica gel column chromatography (the mobile phase was a mixture of petroleum ether and ethyl acetate with a volume ratio of 1:1), and the product was subjected to vacuum After drying, 10 mg of dark red solid was obtained, which was tripterine derivative c, and the yield was 20%.
[0040] The spectral analysis of the tripterine derivative c is as follows: M.p.183°C, 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):6.98(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com