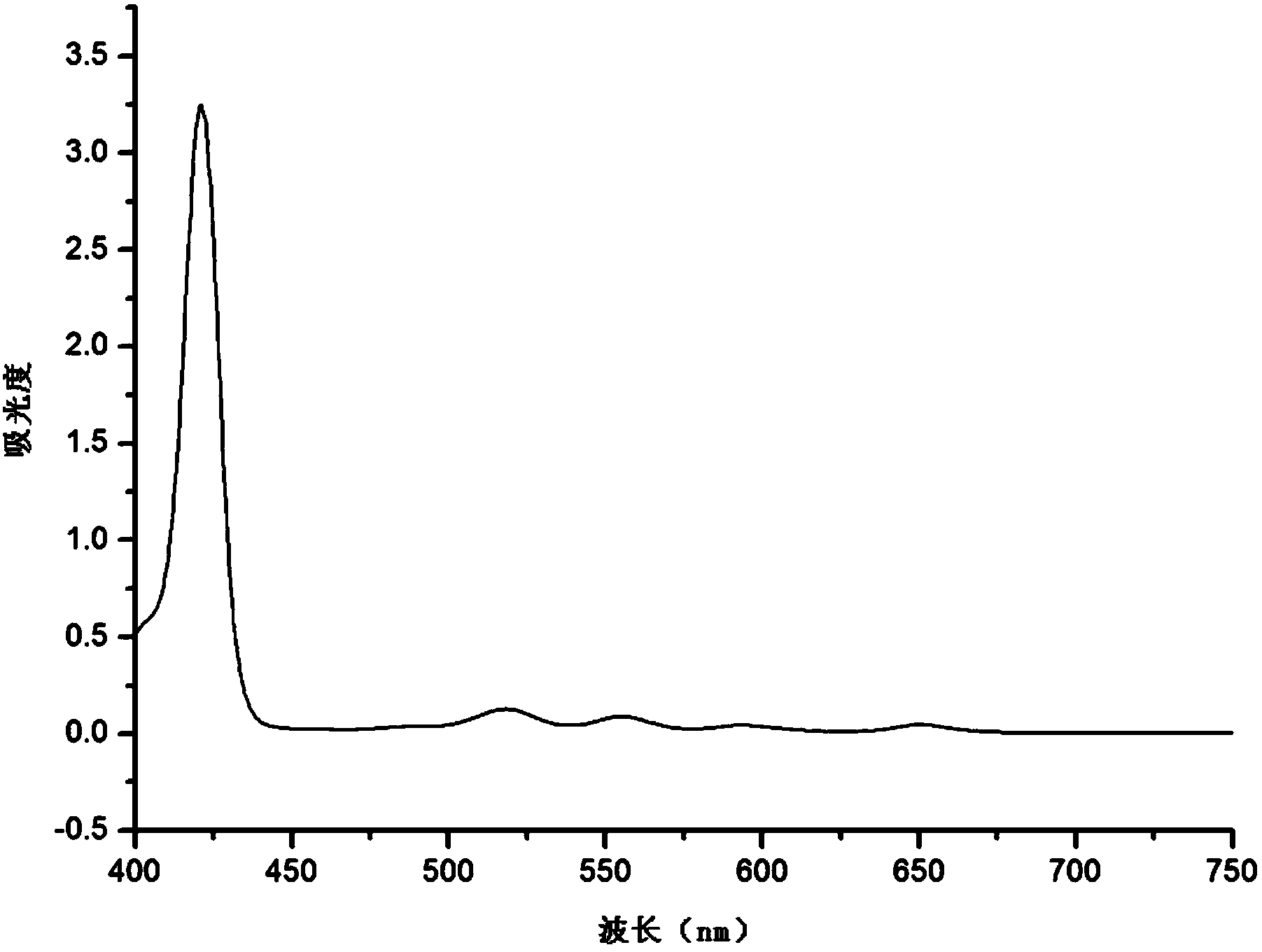
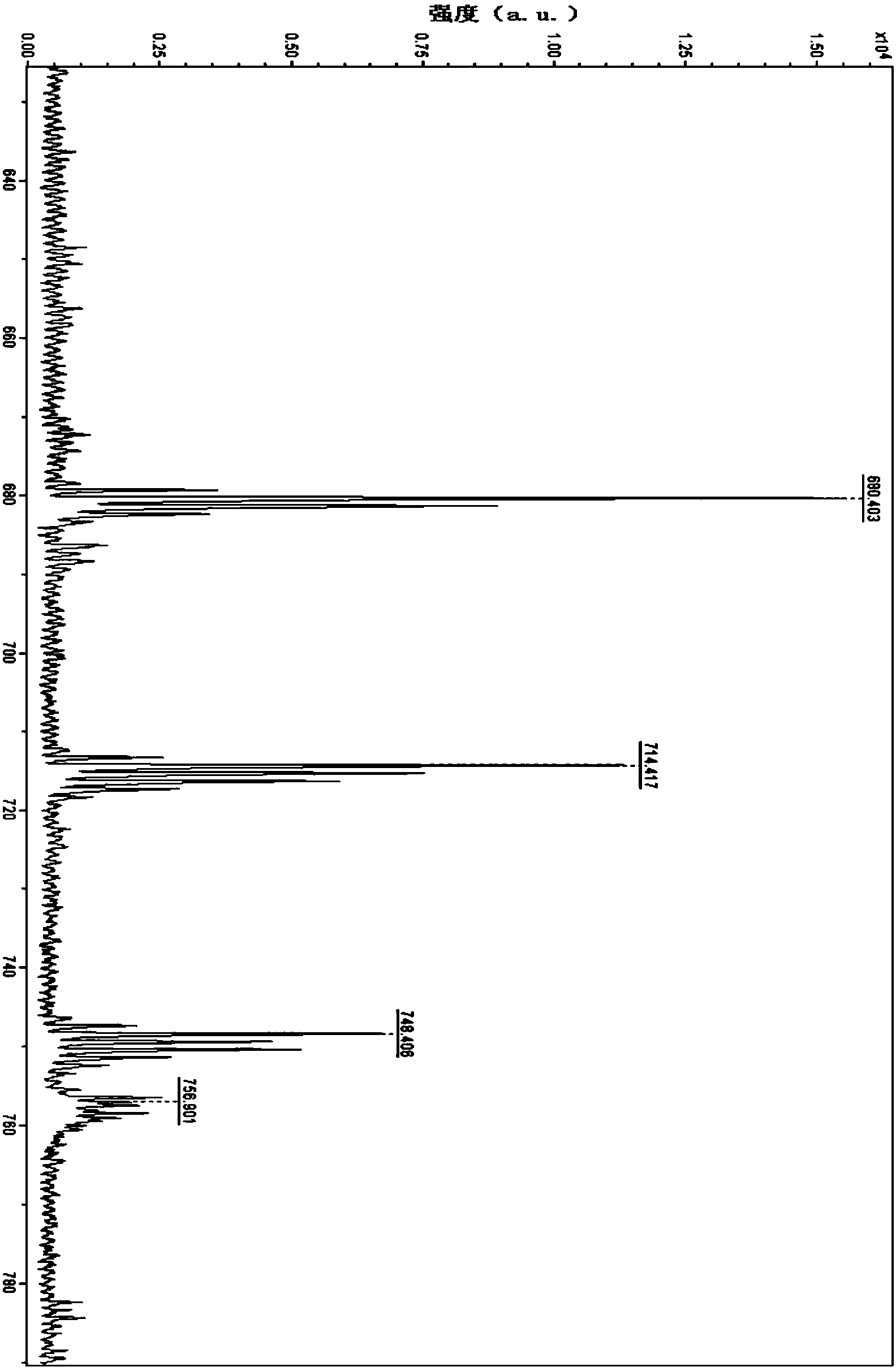
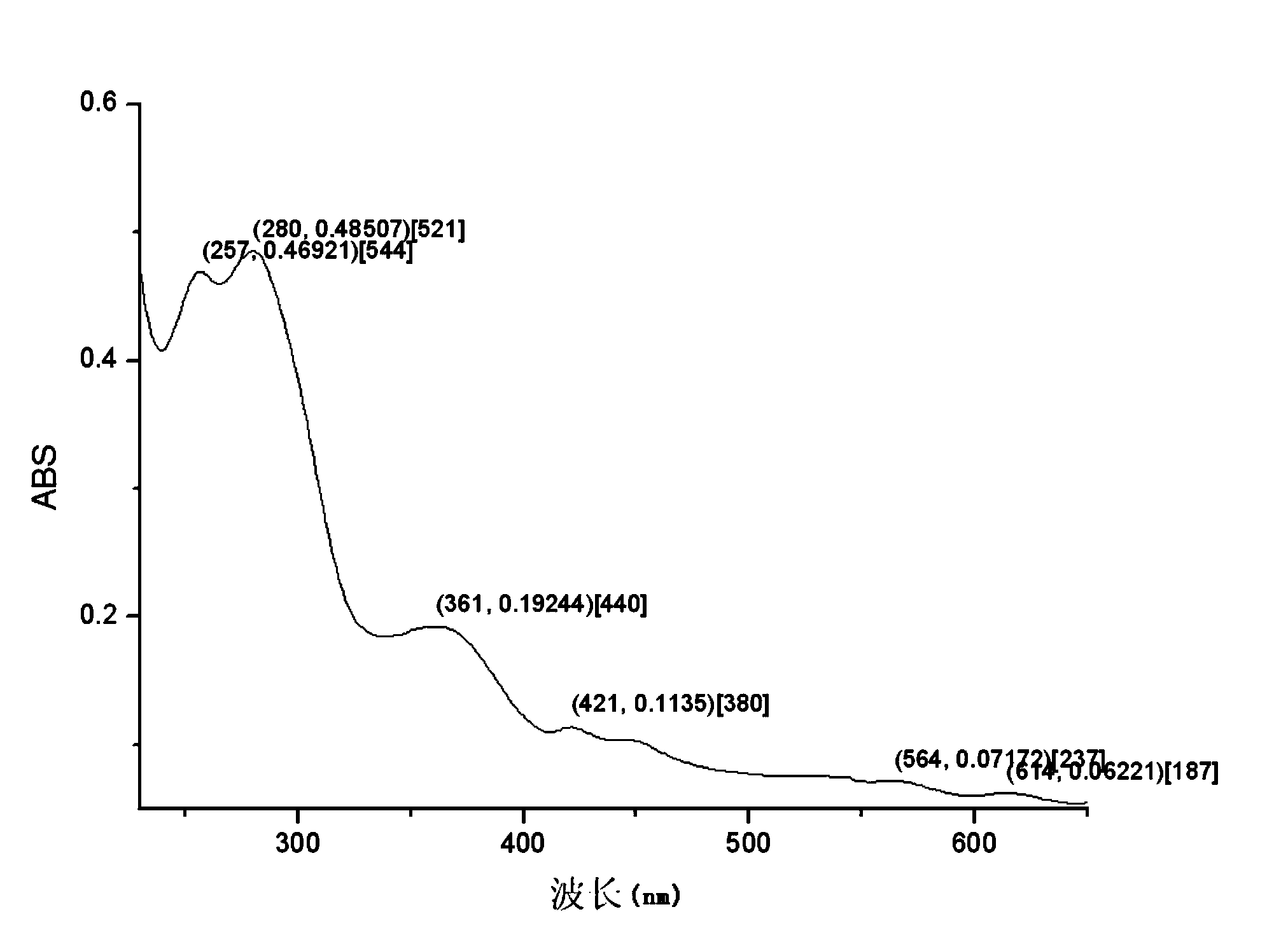
Preparation method of targeted ferroferric oxide-porphyrin containing composite nanoparticles
A technology of composite nanoparticles and ferric oxide, which is applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of weak fluorescence signal intensity of photosensitizers and tumor tissue selection. The problem of low sexual uptake and high cumulative effect of photosensitizers can achieve the effect of inhibiting tumor metastasis, improving immunity and reducing diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific embodiment one: this embodiment has the preparation method of targeted iron ferric oxide-porphyrin composite nanoparticles, specifically according to the following steps:
[0027] 1. Using FeCl 3 ·6H 2 O to prepare FeCl with a concentration of 0.1mol / L 3 solution, using FeSO 4 4H 2 O to prepare FeSO with a concentration of 0.1mol / L 4 solution;
[0028] Under the condition of nitrogen protection and mechanical stirring, according to Fe 3+ with Fe 2+ The molar ratio is 2:1, and 100mL of FeCl with a concentration of 0.1mol / L 3 solution and 50 mL of FeSO with a concentration of 0.1 mol / L 4 The solutions are mixed to obtain FeCl 3 and FeSO 4 The mixed solution of the mixed solution was adjusted to a pH value of 5 by hydrochloric acid with a concentration of 1 mol / L, and then an aqueous ammonia solution with a mass concentration of 25% was added until a black precipitate appeared in the mixed solution, and the stirring was continued for 1 hour to obtain a sol...
specific Embodiment approach 2
[0035] Embodiment 2: The difference between this embodiment and Embodiment 1 is that in Step 1, the mixture of double distilled water and ethanol with a volume ratio of 1:1 is used for washing. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0036] Embodiment 3: This embodiment is different from Embodiment 1 in that: 100 mL of double distilled water is added in step 2. Others are the same as in the first embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com