Enzyme linked immunosorbent assay kit used for detecting content of heavy metal copper ions in sample
An enzyme-linked immunosorbent reagent and copper ion technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that it is difficult to adapt to the requirements of rapid customs clearance of products, and cannot use on-site detection, etc., to achieve easy promotion, easy promotion and application, and judgment image effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, the preparation of copper ion chelate, polyclonal, monoclonal antibody
[0026] 1.1 Transformation and identification of copper ion chelate
[0027]Weigh 76.7 mg of copper nitrate with a purity of 99.99% and dissolve it in 100 μL of high-grade concentrated nitric acid. After fully dissolving, add ultrapure water to make the final volume 1 mL to form a copper solution with a concentration of 409 mmoL / L. Weigh 10mg EDTA and dissolve in 10mM HBS buffer to prepare EDTA solution. After mixing the two evenly, adjust the pH value to 6.0 with NaOH, and react on a shaker at room temperature for 24 hours to form Cu 2+ - EDTA chelate solution.
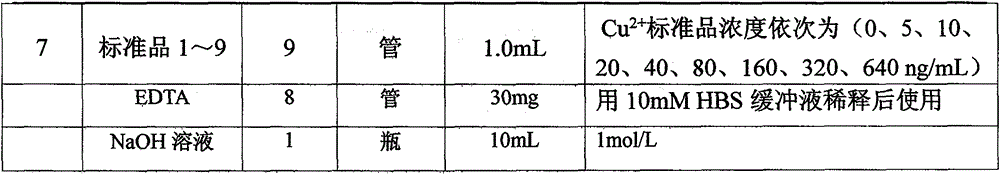
[0028] 100 μg / mL of Cu 2+ The standard stock solution is diluted with 2% nitric acid to form a concentration gradient of 0 μg / mL, 0.25 μg / mL, 0.5 μg / mL, 1 μg / mL, 2 μg / mL, and 4 μg / mL. The instrument software automatically draws a standard curve and obtains a linear Regression equation; the sample solution was diluted 50 t...
Embodiment 2
[0036] Embodiment 2, preparation of copper ion detection ELISA kit
[0037] 2.1 detection reaction principle of immunoassay kit
[0038] The immunoassay kit selects the appropriate coating antigen concentration to coat the microtiter plate, and the detection kit made after blocking with heterologous protein can be used for the detection of samples. Take the treated samples to be tested and react with the provided polyclonal or monoclonal antibodies as required. The incompletely bound antibodies bind to the sites on the microtiter plate, and the goat antibody labeled with horseradish peroxidase Mouse or rabbit anti-mouse secondary antibody binding reaction, through color development to reflect the color development, observe the results, or use a microplate reader to read the degree, and calculate the content of the analyte according to the results. The judgment of the result is compared with the provided negative and positive controls. If the color depth is exactly the same as...
Embodiment 3
[0052] Embodiment 3, the performance of copper ion ELISA kit
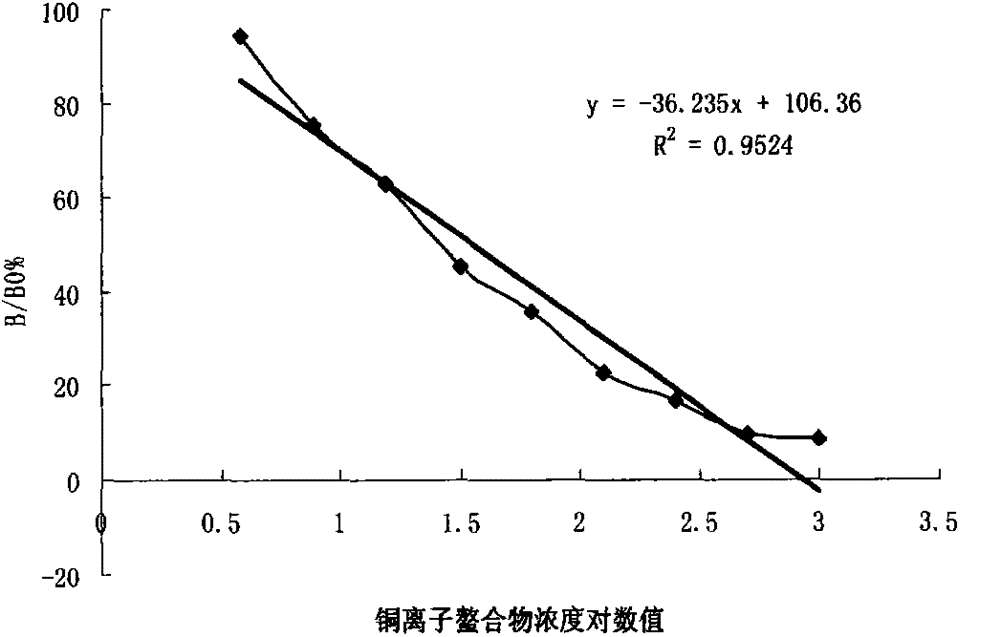
[0053] 3.1 Sensitivity determination according to the minimum detection limit of blocking ELISA is B / B 0 %=120%, calculate the sensitivity of the kit according to the curve regression equation, and determine the detection limit. As a result, the detection limit was 0.42 μg / L. According to the results obtained by blocking ELISA, B / B 0 %=10%~90% is the detection range, according to the curve regression equation, the detection range of the kit is 2.83~456.04μg / L.
[0054] 3.2 Accuracy determination of Cu 2+Standards were added to soil and tap water at final concentrations of 5, 10, 20, and 40 μg / L, respectively, and six replicates were set up. The accuracy was determined by recovery and coefficient of variation (CV). The results are shown in Table 2. The recovery rate of soil is 77.25%-82.3%, with an average of 80.56%, and the coefficient of variation (CV) is 9.5%-13.7%; the recovery rate of tap water is 80.8%-88...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com