Preparation method of ((4-p-fluorophenyl-6-isopropyl-2-(N-methylmethanesulfonamide)-5-pyridyl) methyl)triphenyl phosphonium salt
A technology of methylmethanesulfonamide and p-fluorophenyl, which is applied in the field of preparation of -5-pyridyl)methyl)triphenylphosphonium salt, can solve the problem of unsuitable large-scale production, high price of monomethylguanidine, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
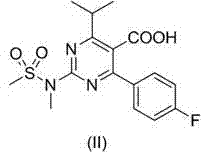
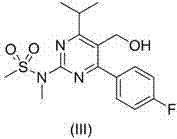
[0037] Embodiment 1, 4-p-fluorophenyl-5-hydroxymethyl-6-isopropyl-2-( N Synthesis of -methylmethylsulfonamido)pyrimidine (III)
[0038] Under nitrogen protection, sodium borohydride (0.38 g), dry tetrahydrofuran (40 mL) and glass beads (10 mL) were placed in a dry reaction flask, and trimethylchlorosilane (2.1 g) was added dropwise with stirring at room temperature After the addition, the temperature was raised to 60°C and stirred for 3h, then 4-p-fluorophenyl 6-isopropyl-2-( N -Methylmethanesulfonamido) pyrimidine-5-carboxylic acid (1.83 g) suspension in tetrahydrofuran (5 mL), after the addition was complete, keep stirring for 24 hours. After the reaction is complete, cool the reaction liquid to 0~5°C, slowly add saturated ammonium chloride solution dropwise, after the addition is complete, stir for 15 min, extract with dichloromethane, wash with 10% sodium carbonate solution, dry over anhydrous sodium sulfate, and reduce pressure The solvent was recovered to dryness, and ...
Embodiment 2
[0039] Embodiment 2, 4-p-fluorophenyl-5-hydroxymethyl-6-isopropyl-2-( N Synthesis of -methylmethylsulfonamido)pyrimidine (III)
[0040] Under nitrogen protection, potassium borohydride (0.54 g), dry tetrahydrofuran (40 mL) and steel beads (10 mL) were placed in a dry reaction flask, and trimethylchlorosilane (1.1 g) was added dropwise with stirring at room temperature, After the addition is complete, heat to 65°C and stir at reflux for 3h, then add 4-p-fluorophenyl 6-isopropyl-2-( N -Methylmethanesulfonamido)pyrimidine-5-carboxylic acid (1.83 g) in tetrahydrofuran (5 mL) suspension, after addition, keep stirring for 30 h. After the reaction is complete, cool the reaction liquid to 0~5°C, slowly add saturated ammonium chloride solution dropwise, after the addition is complete, stir for 15 min, extract with dichloromethane, wash with 10% sodium carbonate solution, dry over anhydrous sodium sulfate, and reduce pressure The solvent was recovered to dryness, and a solid was preci...
Embodiment 3
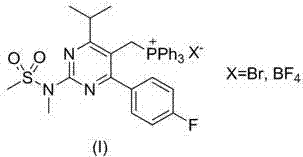
[0041] Embodiment 3, ((4-p-fluorophenyl-6-isopropyl-2-( N -Synthetic (X=BF) of triphenylphosphonium fluoroborate (I) of -methylmethylsulfonyl amido)-5-pyridyl)methyl) 4 )
[0042] Dissolve III (0.88 g) and triphenylphosphonium fluoroborate (0.88 g) in acetonitrile (20 mL), heat to 81°C and stir under reflux for 24 hours. After the reaction is complete, concentrate to dryness under reduced pressure to obtain a white foamy solid I(X=BF 4 , 1.67 g, 97%), 1 H NMR (400MHz, CDCl 3 ): δ 7.74( t, J =7.8 Hz, 3H), 7.54 ( td, J =8, 3.2 Hz, 6H), 7.26( t, J =6.8 Hz, 2H), 7.17(dd, J =12.8, 7.8 Hz, 6H), 6.98( t, J =8.4 Hz, 2H), 5.17 ( d, J =12.4 Hz, 2H), 3.48 ( s, 3H), 3.43 ( s, 3H), 2.73( sept. , J =6.4 Hz, 1H), 0.88(br, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com