Recombinant adenovirus as well as preparation method and application thereof
A recombinant adenovirus and adenovirus technology, applied in the field of molecular biology, can solve the problems of not being able to become an immunotherapy method, inducing severe reactions, and inconvenient administration, etc., and achieve good application prospects, less frequent administration, and high compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
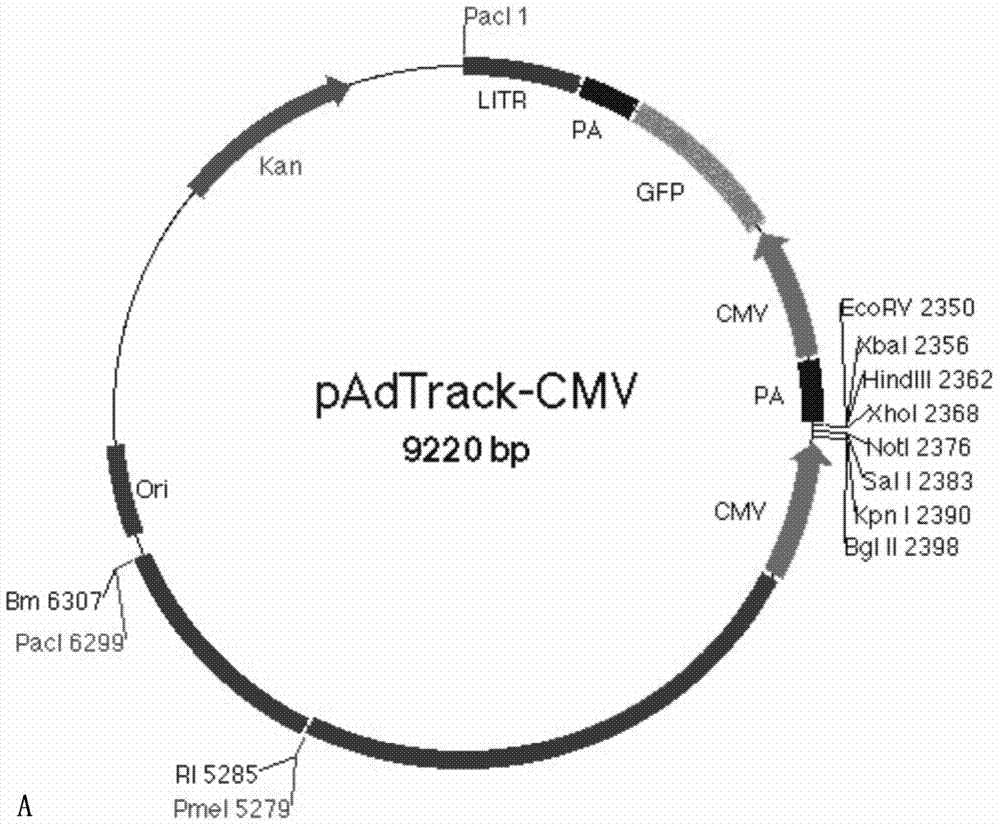
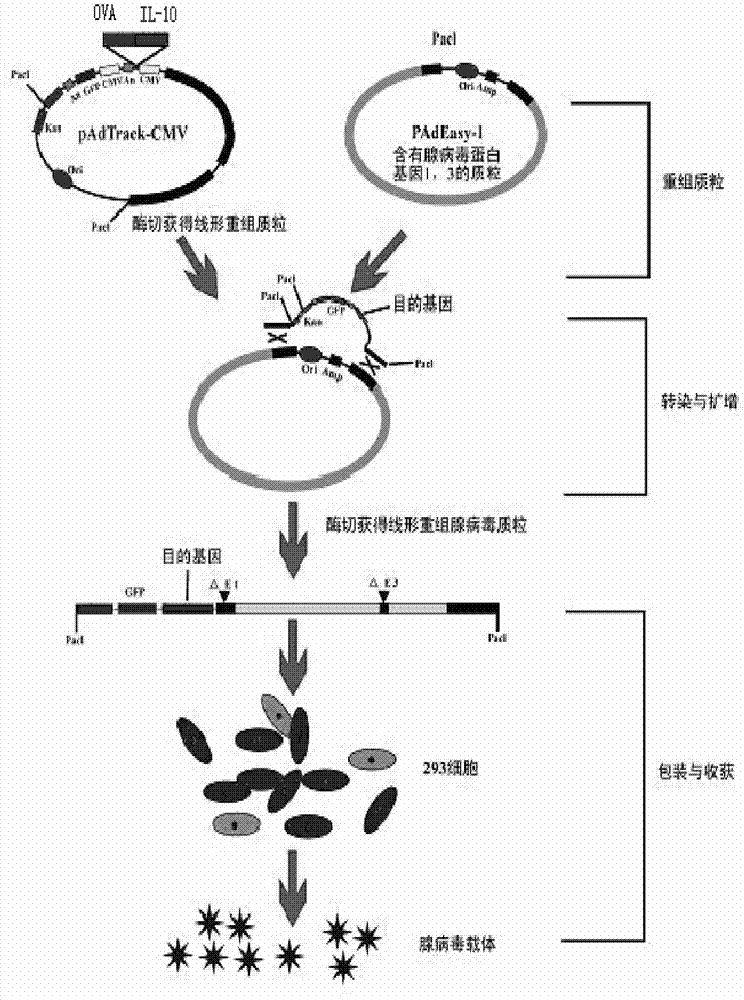
[0043] Example 1: Inserting the hIL-10 gene into the shuttle plasmid to construct the recombinant shuttle plasmid pAdTrack-hIL-10
[0044] 1. PCR amplification of hIL-10 gene
[0045] (1) Isolation of peripheral blood mononuclear cells from healthy people
[0046] Take 10 ml of peripheral venous blood from healthy people, anticoagulate with heparin sodium, add an equal volume of 0.9% sodium chloride to dilute the anticoagulated blood. Then take 10 ml of the lymphocyte separation solution stored at 4°C in the dark and add it to the bottom of a sterile centrifuge tube, and warm it up to room temperature. Use a Pasteur pipette to draw 20 ml of the diluted blood sample, and spread it slowly along the tube wall on top of the lymphocyte separation medium, trying to avoid disturbing the interface of the liquid layer. The horizontal rotor was centrifuged at 2000 rpm for 20 minutes at room temperature. After centrifugation, it can be seen that the liquid in the centrifuge tube is ...
Embodiment 2
[0080] Example 2: OVA gene was inserted into the shuttle plasmid to construct the recombinant shuttle plasmid pAdTrack-hIL-10-OVA
[0081] 1. PCR amplification of OVA gene
[0082] Firstly, the total RNA of chicken fallopian tube epithelial cells was extracted by TRIzol reagent, and cDNA was synthesized by reverse transcription with Oligo dT (oligomeric T) as a primer. According to the OVA gene sequence (GenBank accession number AY223553.1), primers were designed using Primer Premier5 software. These include the upstream Xho I and downstream Xba I restriction sites (underlined), and the primer sequences were synthesized by Shanghai Sangong.
[0083] Amplification primers are as follows:
[0084] Upstream primer: 5'TAC CTCGAG ATGGGCTCCATCGGTG3' (SEQ ID No. 8)
[0085] Downstream primer: 5'GCGC TCTAGA TTAAGGGGAAACACA3' (SEQ ID No. 9)
[0086] First, pre-denaturation at 95°C for 5 minutes; followed by thermal cycling, the reaction conditions are: denaturation at 95°C for...
Embodiment 3
[0105] Example 3: Construction of recombinant adenovirus plasmid (homologous recombination method in bacteria)
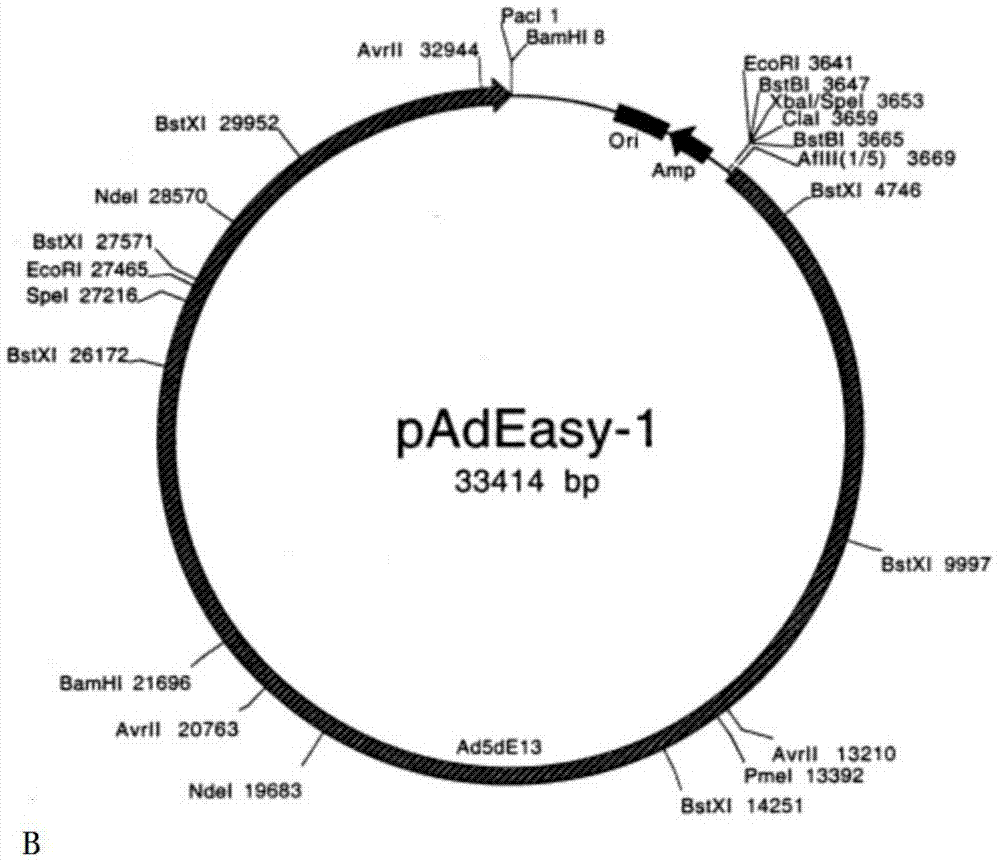
[0106] The linearized recombinant shuttle plasmid pAdTrack-hIL-10-OVA (kanamycin resistance) and the adenovirus backbone plasmid pAdEasy-1 (ampicillin resistance) were co-transformed into BJ5183 bacteria for homologous recombination, containing kanamycin The medium was screened, identified by agarose electrophoresis restriction enzyme digestion and PCR, and the correct recombinant plasmid was named pAd-hIL-10-OVA.
[0107] 1. Preparation of competent state for BJ5183 transformation
[0108] The steps are the same as the above DH5α competent preparation method, except that the culture medium contains 30 μg / ml streptomycin to inhibit the growth of other bacteria.
[0109] 2. Homologous recombination
[0110] Extract the pAdTrack-hIL-10-OVA plasmid with the Omega Plasmid Extraction Kit, and digest it with the restriction endonuclease Pme Ⅰ to make it linear; purify t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com