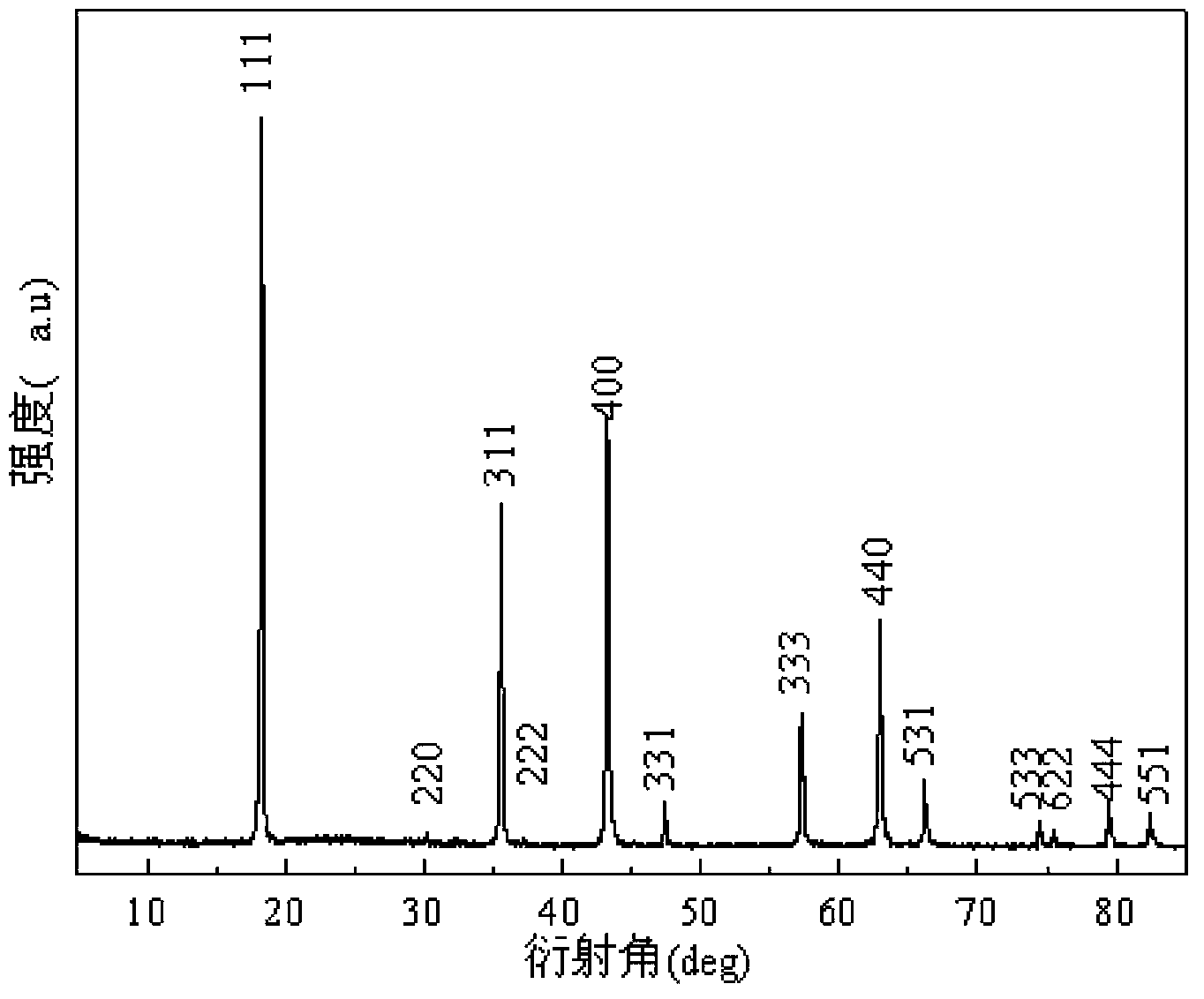
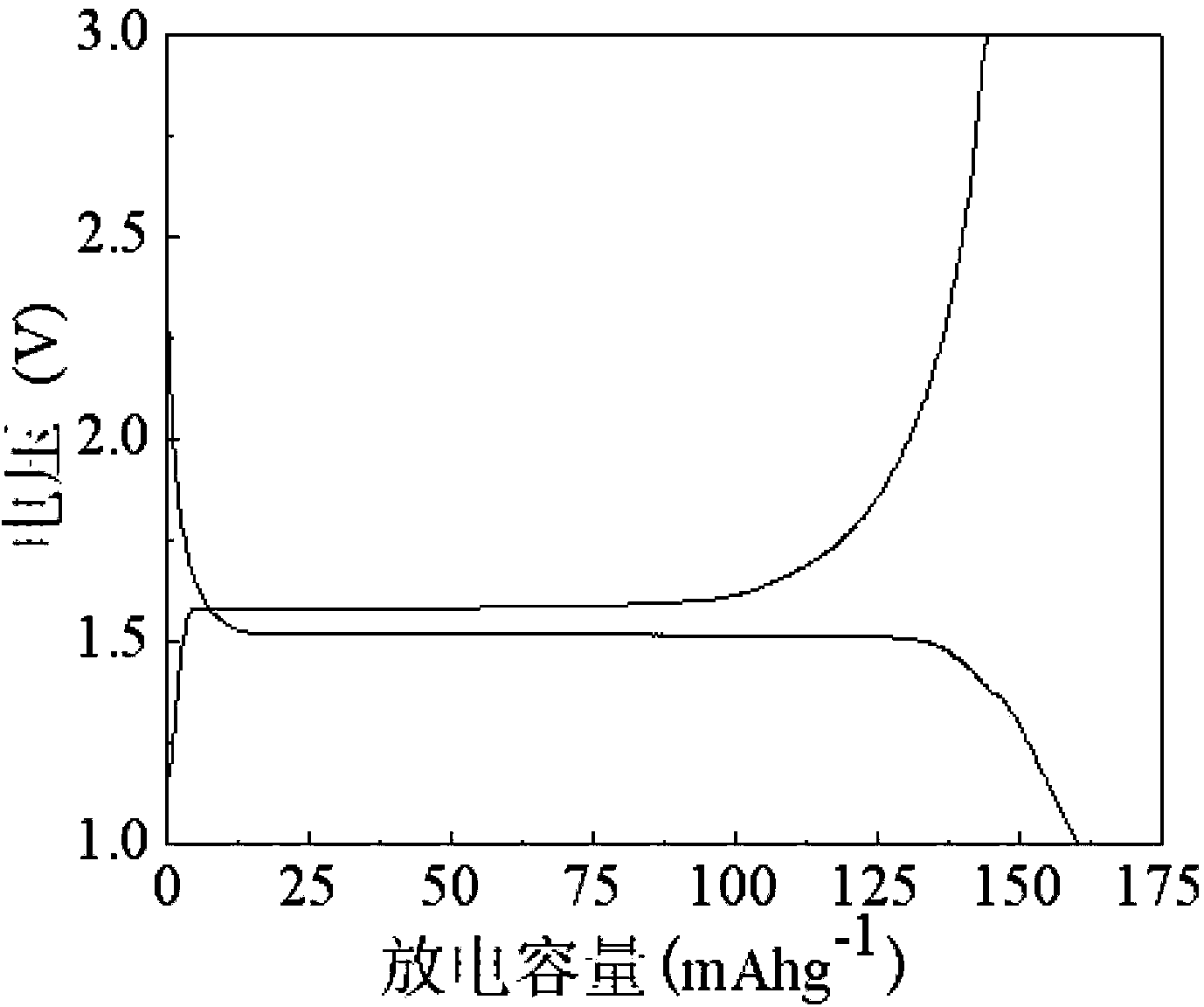
Preparation method of yttrium-containing lithium ion battery cathode material lithium titanate carbon-coated composite material
A technology of lithium-ion batteries and carbon composite materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of electrolyte consumption, low initial Coulombic efficiency, battery explosion, and coarse particles, etc., to improve high-rate performance, Effect of improving electrical conductivity and improving particle shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesize 12g of Li 4 Y 0.3 Ti 4.7 o 12 , according to the molar ratio Li:Ti:Y=50:47:3, weigh 9.5555g of anatase TiO 2 (analytical pure), the lithium acetate of 12.9769g (analytical pure), the Y of 0.8622g 2 o 3 (Analytical pure) and 1.8g of phenolic resin, join in the ball mill jar, then add the agate ball of 75g and the dehydrated alcohol of 70ml, seal the ball mill jar, ball mill with the rotating speed of 350r / min on the ball mill for 8 hours, obtain mixing of mixed slurry. Transfer the slurry to a drying dish, put it in an oven and dry it at 100°C to obtain the precursor. Put the precursor powder in a tube furnace, and under the protection of argon, raise the temperature to 350°C at a rate of 100°C / hour and keep it warm for 6 hours to decompose the raw material initially. After the temperature drops to 20-50°C, the calcined powder is obtained. body. Put the calcined powder into a ball mill jar, add 3g of soluble starch, then add 36g of agate balls and 55ml...
Embodiment 2
[0024] Synthetic Li 4 Y 0.1 Ti 4.9 o 12 / C composite material, where Li 4 Y 0.1 Ti 4.9 o 12 The mass is 8g, according to the molar ratio Li:Ti:Y=50:49:1, weigh 6.7591g of anatase TiO 2 (analytical pure), the lithium acetate of 8.7658g (analytical pure), the Y of 0.2925g 2 o 3 (analytically pure) and 1.44g citric acid, join in the ball mill jar, then add the agate ball of 50g and the dehydrated alcohol of 40ml, seal the ball mill jar, ball mill 2 hours with the rotating speed ball mill of 400r / min on the ball mill, obtain the homogeneously mixed Mix slurry. Transfer the slurry to a drying dish, put it into an oven and dry it at 120°C to obtain a precursor. Put the precursor powder in a tube furnace, and under the protection of argon, raise the temperature to 400°C at a rate of 100°C / hour and keep it warm for 5 hours to decompose the raw material initially. After the temperature drops to 20-50°C, the calcined powder is obtained. body. Put the calcined powder into a b...
Embodiment 3
[0026] Synthetic Li 4 Y 0.1 Ti 4.9 o 12 / C composite material, where Li 4 Y 0.1 Ti 4.9 o 12 The mass is 10g, according to the molar ratio Li:Ti:Y=50:49:1, weigh 8.4489g of anatase TiO 2 (analytical pure), the lithium acetate of 10.9572g (analytical pure), the Y of 0.3656g 2 o 3 (analytical pure) and 2.6389g sucrose, join in the ball mill jar, then add the agate ball of 67g and the dehydrated alcohol of 50ml, seal the ball mill jar, ball mill with the rotating speed of 400r / min on the ball mill for 10 hours, obtain the mixed mixture slurry. Transfer the slurry to a drying dish, put it in an oven and dry it at 100°C to obtain the precursor. Put the precursor powder in a tube furnace, under the protection of argon, raise the temperature to 500°C at a heating rate of 180°C / hour and keep it warm for 6 hours to decompose the raw material initially. After the temperature drops to 20-50°C, the calcined powder is obtained body. Put the calcined powder into a ball mill jar, ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com