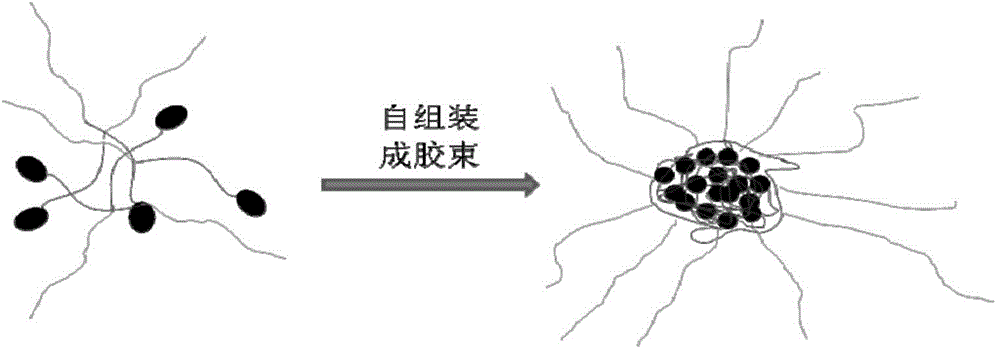
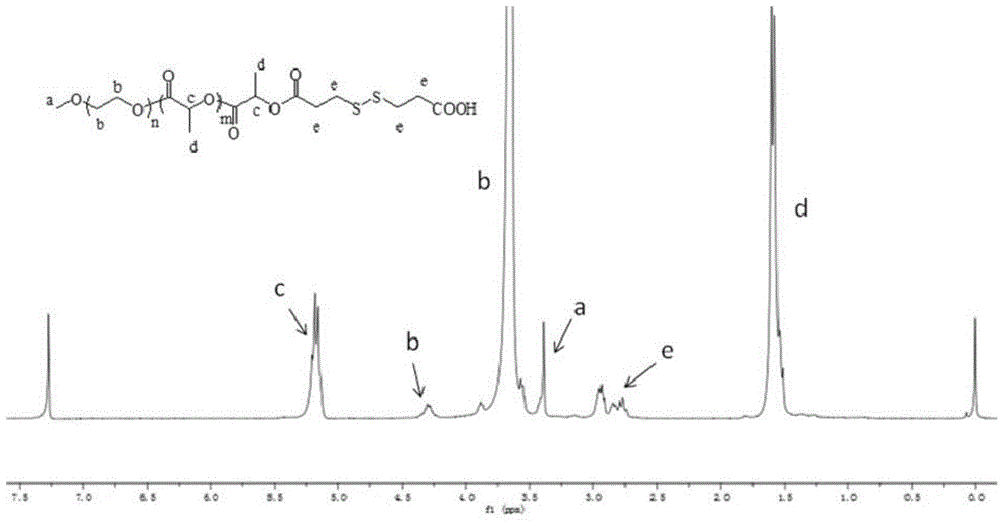
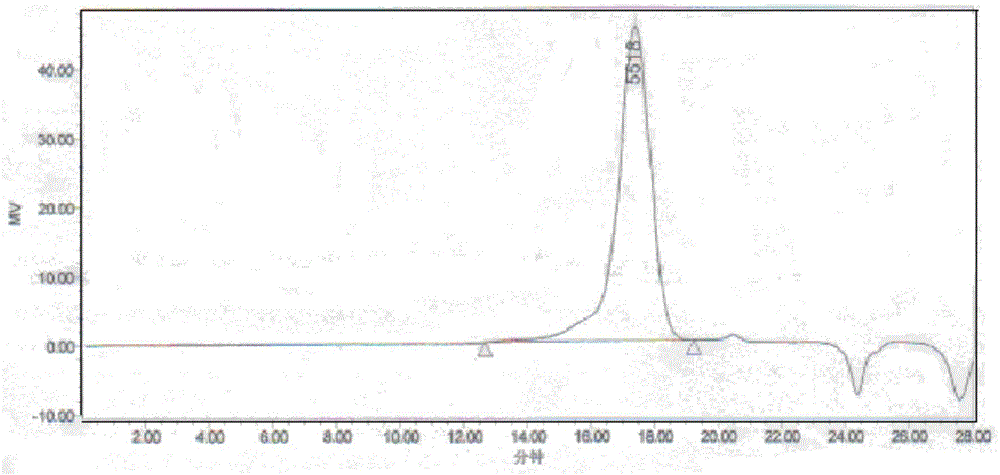
Paclitaxel polymer bonding drug and preparation method thereof
A paclitaxel and polymer technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of bond breaking sensitivity and unsatisfactory regioselectivity, and achieve good affinity Water and biocompatibility, efficient targeting, avoiding phagocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the polymer containing terminal carboxyl group is:
[0035] (A) Using polyethylene glycol monomethyl ether or polyethylene glycol as an initiator, the aliphatic cyclic ester monomer is ring-opened and polymerized under the action of a catalyst to obtain an amphiphilic polymer;
[0036] (B) reacting the amphiphilic polymer with the diacid containing disulfide bonds in the first organic solvent under the action of an organic base and a condensing agent to obtain a carboxyl-terminated polymer.
[0037] The polymer-bonded drug of paclitaxel of the present invention includes a carboxyl-terminated polymer and paclitaxel, and the paclitaxel and the carboxyl-terminated polymer are bonded together through esterification. The preparation method of the polymer containing terminal carboxyl group is:
[0038] First, using polyethylene glycol monomethyl ether or polyethylene glycol as an initiator, the aliphatic cyclic ester monomer is ring-opened and polym...
Embodiment 1
[0052] Add 10 g of polyethylene glycol monomethyl ether (MPEG) with a molecular weight of 2000 into a dry reaction bottle equipped with a water separator and a stirring device, replace the argon for 3 times, remove water by azeotropic anhydrous toluene, and remove large After removing part of the toluene, change to a decompression device to remove the remaining toluene, and dry it under vacuum at 80°C for 6 hours. After returning to room temperature, add 6 g of recrystallized lactide under the protection of an inert gas, heat the system to 100 ° C, the reactant is in a molten state, add 8 mg of stannous octoate, stir well, and react at 130 ° C for 24 hours. The product was dissolved in an appropriate amount of dichloromethane, precipitated with ether, and dried in vacuo to obtain 12.8 g of a white powder product with a yield of 80%. The NMR test result of the obtained polymer shows that the molecular weight of the polymer MPEG-PLA is 2000-1000, and the GPC test result shows a ...
Embodiment 2
[0056] (1) Add 10 g of dihydroxypolyethylene glycol (PEG) with a molecular weight of 2000 into a dry reaction flask equipped with a water separator and a stirring device, replace the argon gas 3 times, and azeotropically remove water with anhydrous toluene. After most of the toluene was removed, the remaining toluene was removed by a decompression device, and vacuum-dried at 80° C. for 6 hours. After returning to room temperature, add 6 g of recrystallized lactide under the protection of an inert gas, heat the system to 100 ° C, the reactant is in a molten state, add 8 mg of stannous octoate, stir well, and react at 130 ° C for 24 hours. The product was dissolved in an appropriate amount of dichloromethane, precipitated with ether, and dried in vacuo to obtain 12.8 g of a white powder product with a yield of 80%. The NMR test result of the obtained polymer shows that the molecular weight of the polymer PLA-PEG-PLA is 500-2000-500, and the GPC test result shows a single peak. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com