Novel method for preparing taurine
A technology of taurine and bisulfite, applied in the preparation of sulfonic acid, organic chemistry and other directions, can solve the problems of high energy consumption, potential safety hazards, etc., and achieve the effects of high yield, convenient operation and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthetic method one of 2-nitroethanol
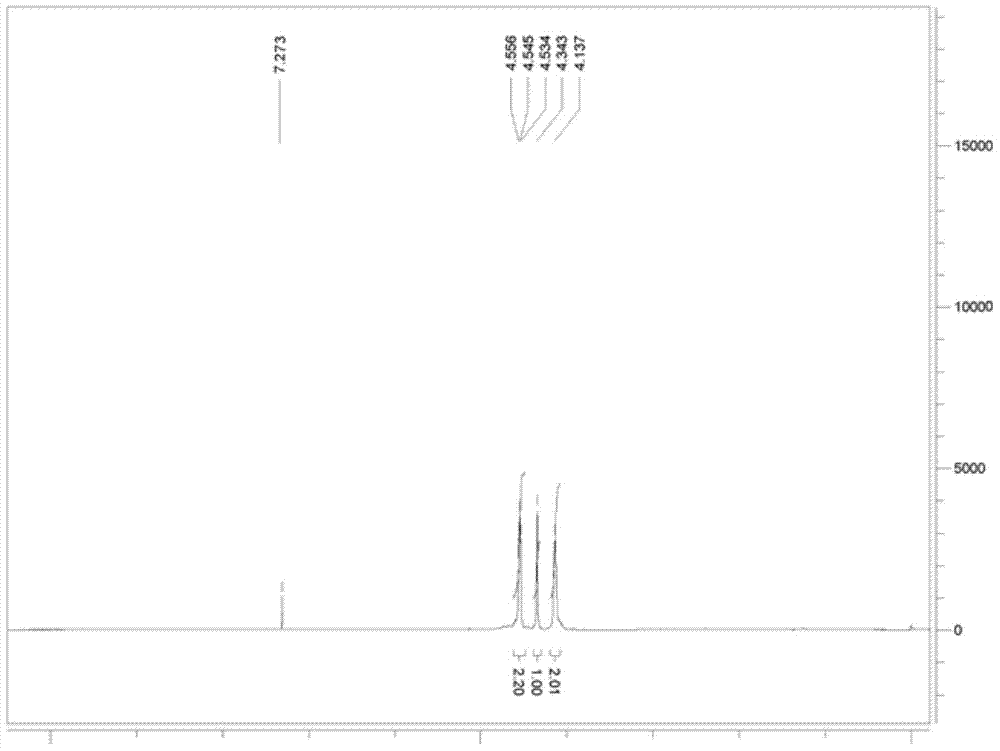
[0035] At 30°C, add paraformaldehyde (50g, 1.56mol), nitromethane (285.6g, 4.7mol, 253mL), 2.5L of acetic acid into a 2000mL reactor and start stirring, add anhydrous potassium acetate (5g, 0.05mol ), continue to stir for 3h, then heat up and reflux for 30min. Unreacted nitromethane and solvent acetic acid were recovered by distillation under reduced pressure to obtain 139.8 g of red oil with a yield of 92.1%, which was directly carried out to the next step without purification. 1 H NMR (400MHz, CDCl 3 ): δ4.55(t, 2H, J=4.4Hz), 4.34(s, 1H), 4.14(s, 2H).
Embodiment 2
[0036] Embodiment 2: the synthetic method two of 2-nitroethanol
[0037] At room temperature, under stirring conditions, nitromethane (762.5g, 12.5mol, 675mL), paraformaldehyde (100g, 3.12mol), sodium hydroxide (3.2g, 0.08mol) were added to a 20L reactor, Methanol 8L continued to stir for 5h, then warmed to reflux for 2h. Unreacted nitromethane and solvent methanol were recovered by distillation under reduced pressure to obtain 286.6 g of a light yellow oil with a yield of 94.6%, which was directly carried out to the next step without purification.
Embodiment 3
[0038] Embodiment 3: the synthetic method one of taurine
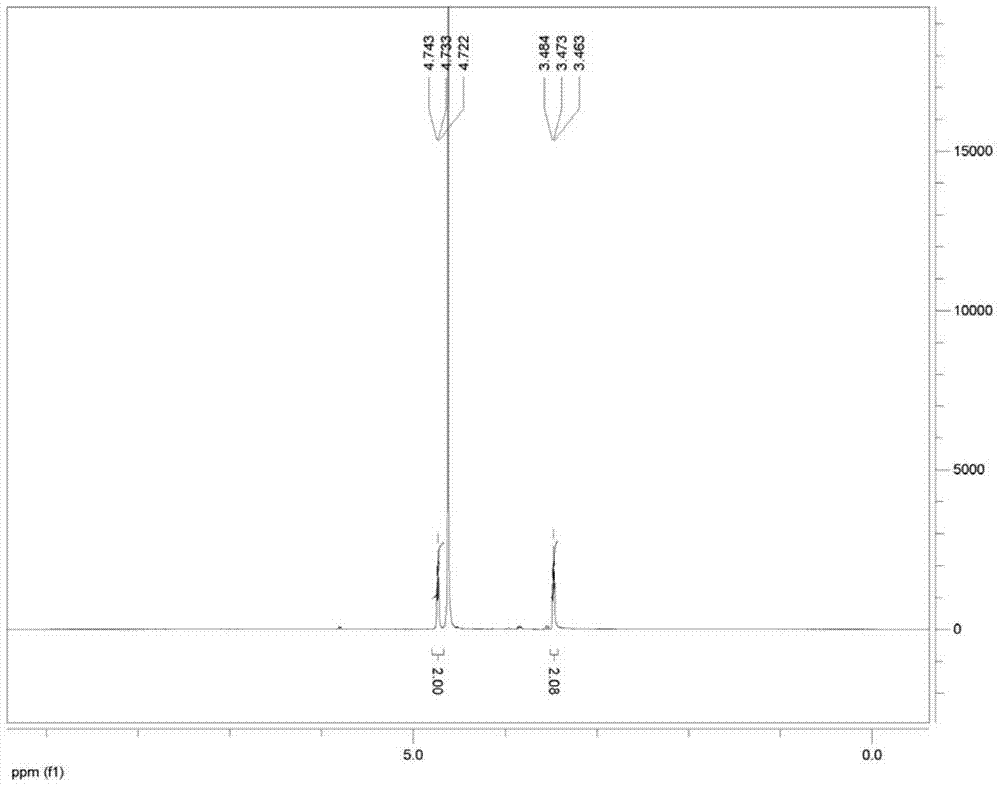
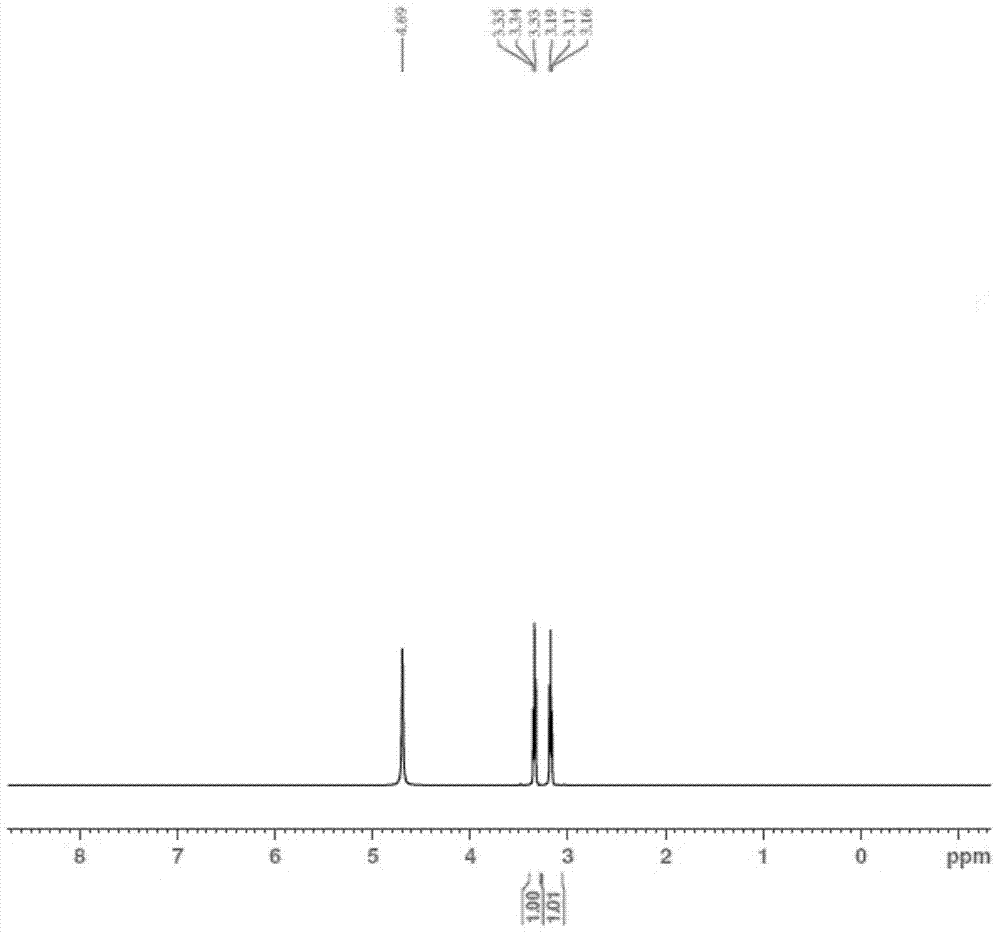
[0039] At 20°C, add 500mL of 10% aqueous sodium hydroxide solution to a 2000mL reaction kettle, pass in sulfur dioxide gas until the pH value is 5.8, then add nitroethanol (164g, 1.8mol), stir for 18h, [concentrate to obtain nitroethyl Sodium sulfonate white solid, 1 H NMR (400MHz,D 2 O): δ4.73(t, 2H, J=4.0Hz), 3.47(t, 2H, J=4.0Hz)], add Ranney Ni20g, pass into hydrogen, wait for the reaction to be completed, separate the water layer and the catalyst, use concentrated Sulfuric acid was used to adjust the pH value to the equivalence point, and taurine was precipitated. Suction filtration and drying gave 180 g of taurine with a yield of 80% and a melting point of 319°C (dec). 1 H NMR (500MHz,D 2 O): δ3.34(t, 2H, J=5.0Hz), 3.17(t, 2H, J=5.0Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com