Pharmaceutical composition containing recombinant tissue plasminogen activator
A composition and drug technology, applied in the directions of drug combination, recombinant DNA technology, medical preparations containing active ingredients, etc., can solve the problems of increased downstream protein purification work, low yield of expressed cell lines, poor stability of tPA protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
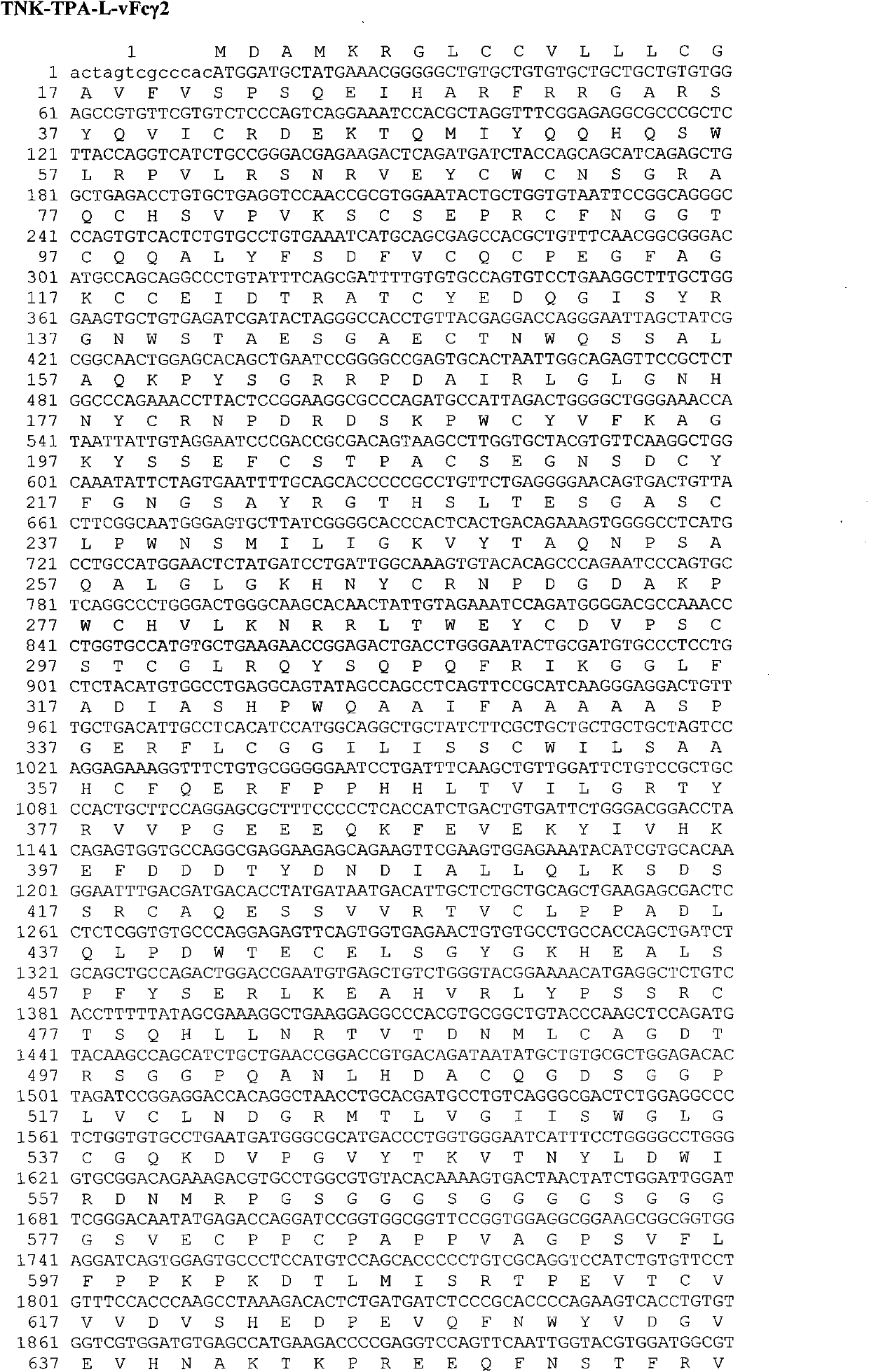
[0078] Example 1. Construction of the gene encoding the TNK-tPA-L-vFcγ fusion protein
[0079] The gene sequence encoding TNK-tPA leading peptide and mature protein is artificially optimized CHO cell preferred codons and obtained by chemical synthesis. In order to facilitate the insertion of the target fragment into the specific site of the expression vector, there is a restriction enzyme endonuclease site at the 5' and 3' ends of the synthesized fragment, respectively SpeI and BamHI. The full-length 1705bp DNA fragment is inserted between the EcoRV restriction enzyme sites in the transfer vector such as pUC57 to obtain an intermediate plasmid whose TNK-tPA gene sequence is verified by DNA sequencing.
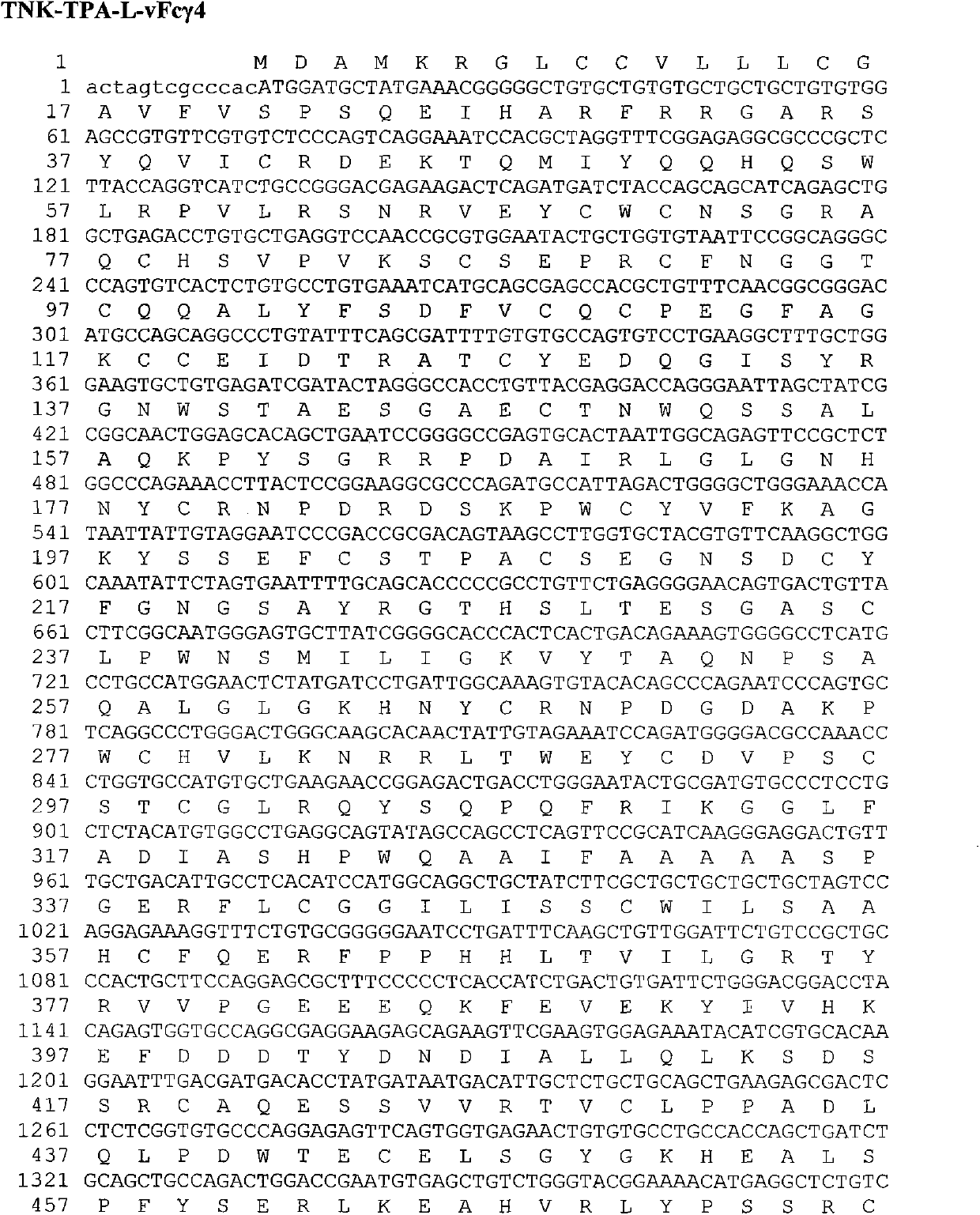
[0080] Flexible peptide linker and human IgG Fc region Fc γ2 variant vFc γ2 (Pro331Ser mutation), Fc γ4 variant vFc γ4 (Ser228Pro and Leu235Ala mutations), Fc γ1 variant vFc γ1 (Leu234Val, Leu235Ala, and Pro331SSer mutations) fusion genes are also artificially optimized C...
Embodiment 2
[0082] Example 2. Expression of Fusion Proteins in Transfected Cell Lines
[0083] Transfection of recombinant expression vector plasmids into mammalian host cell lines to express TNK-tPA-L-vFc γ fusion protein. For stable high-level expression, a preferred host cell line is a DHFR enzyme-deficient CHO-cell (US Patent No. 4,818,679). A preferred method of transfection is electroporation, although other methods including calcium phosphate co-sedimentation, lipofection, and protoplast fusion can also be used. In electroporation, use a Gene Pulser Electroporator (Bio-Rad Laboratories, Hercules, CA) set to a 210V electric field and a 1050 μFd capacitance, in a cuvette of 2 to 3 × 1O 7 Add 20 μg of the plasmid linearized with PvuI to each cell. Two days after transfection, the medium was changed to growth medium containing 0.6 mg / mL G418. Transfectants were screened for resistance to the selective drug using an anti-human IgG Fc ELISA assay. The expression of fusion protein ca...
Embodiment 3
[0085] Example 3. Production of fusion proteins
[0086] The high-yield cell line preferably obtained in Example 2 is first subjected to serum-free acclimation culture in a petri dish, and then transferred to a shake flask for suspension acclimatization culture. After the cells are adapted to these culture conditions, they are then cultured in a 300ml shake flask with feed supplementation or simulated perfusion culture by changing the medium every day. The above-mentioned CHO-derived cell lines were fed-cultured in 100ml shake flasks for 13 days, and the cumulative yield of the expressed recombinant fusion protein was 1.90g / L ( Image 6 ). Between days 6 and 10 of cell culture, the viable cell density can reach a maximum of 22 × 10 6 A / mL. In order to obtain more TNK-tPA-L-vFc recombinant protein, 2000ml shake flask culture can also be selected. In another culture method, the above-mentioned CHO-derived cell line is replaced with a medium in a 100ml volume shake flask every...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com