Solar glass self-cleaning antireflection paint and production method thereof
A technology of solar glass and anti-reflection, which is applied in the direction of coating, etc., can solve the problems that the anti-reflection coating of solar glass cannot adapt to the working environment polluted in the field, the surface of solar anti-reflection glass is easily polluted, and electrostatic adsorption of dust is easy to achieve. Effect of treatment time, reduction of surface resistance, good hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
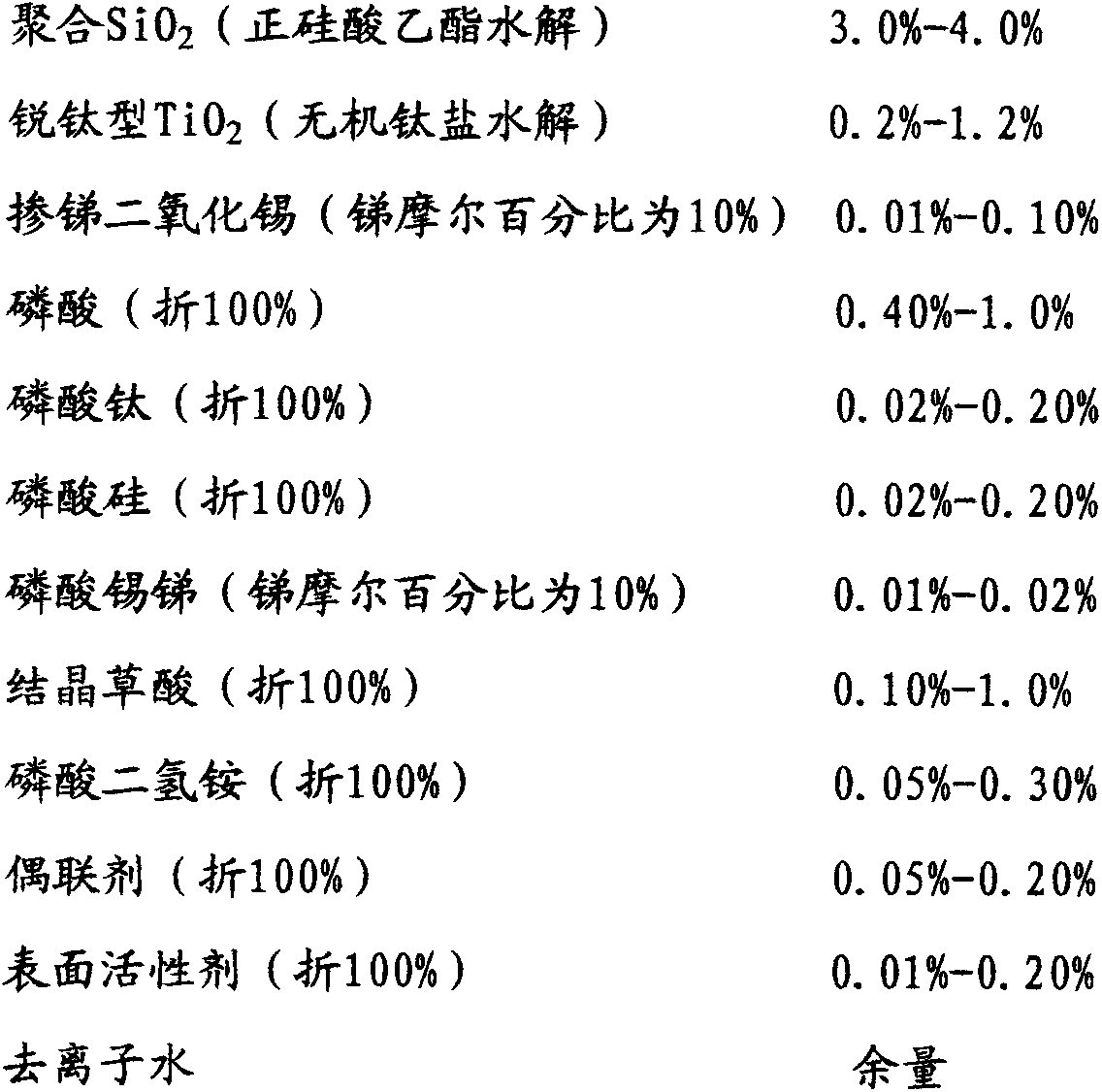
[0038] To a 2000mL four-port reactor equipped with a mechanical stirrer, a thermometer, a dropping funnel, and a condenser, successively add 700 g of ethanol with a mass percentage concentration of 95%, 2.0 g of ammonia water with a mass percentage concentration of 25%, and deionized water. 130g and 160g of ethyl silicate with a mass percentage concentration of 99%, stirred and reacted at 15-30°C for 4-6 hours, left to stand and aged for more than 12 hours to form transparent polymerized SiO 2 Ethanol sol A, the measured average particle size is about 10nm; add 700g of deionized water, and distill 700g of 95% ethanol aqueous solution at 70-75°C to obtain polymerized SiO 2 Aqueous sol B, the measured average particle size is about 10nm; add 30g of dilute phosphoric acid solution with a mass percentage concentration of 20% to adjust the sol to PH2-3 to obtain polymerized SiO 2 Hydrosol C; add anatase nano-TiO with a mass percentage concentration of 5% under stirring 2 100g of p...
Embodiment 2
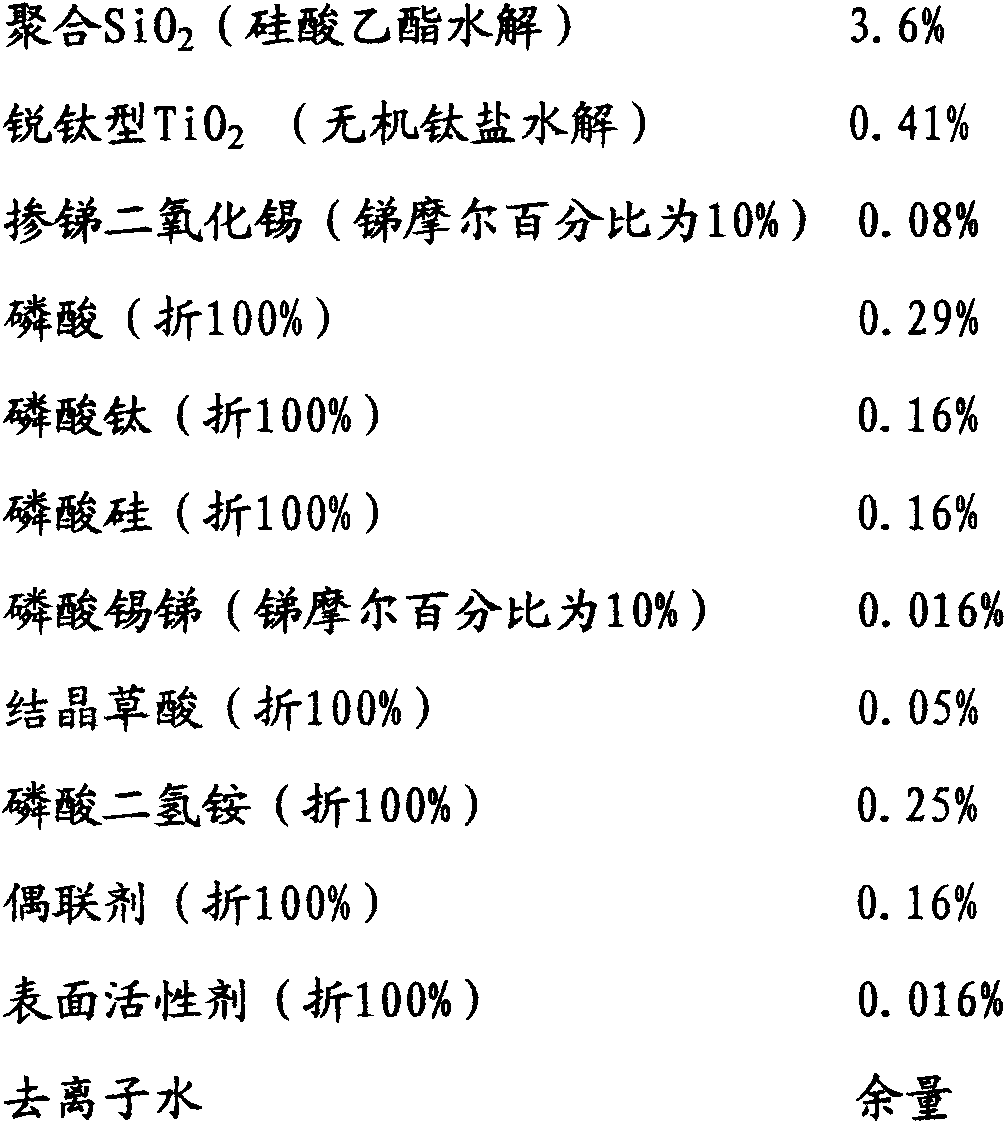
[0042] To a 2000mL four-port reactor equipped with a mechanical stirrer, a thermometer, a dropping funnel, and a condenser, successively add 700 g of ethanol with a mass percentage concentration of 95%, 2.0 g of ammonia water with a mass percentage concentration of 25%, and deionized water. 130g and 160g of orthosilicate ethyl ester with a concentration of 99% by mass, stirred and reacted at 15-30°C for 4-6 hours, left to stand and aged for more than 12 hours to form transparent polymerized SiO 2 Ethanol sol A, the measured average particle size is about 10nm; add 700g of deionized water, and distill 700g of 95% ethanol aqueous solution at 70-75°C to obtain polymerized SiO 2 Aqueous sol B, the measured average particle size is about 10nm; add 30g of dilute phosphoric acid solution with a mass percentage concentration of 20% to adjust the sol to PH2-3 to obtain polymerized SiO 2 Hydrosol C; add anatase nano-TiO with a mass percentage concentration of 5% under stirring 2 50g of...
Embodiment 3
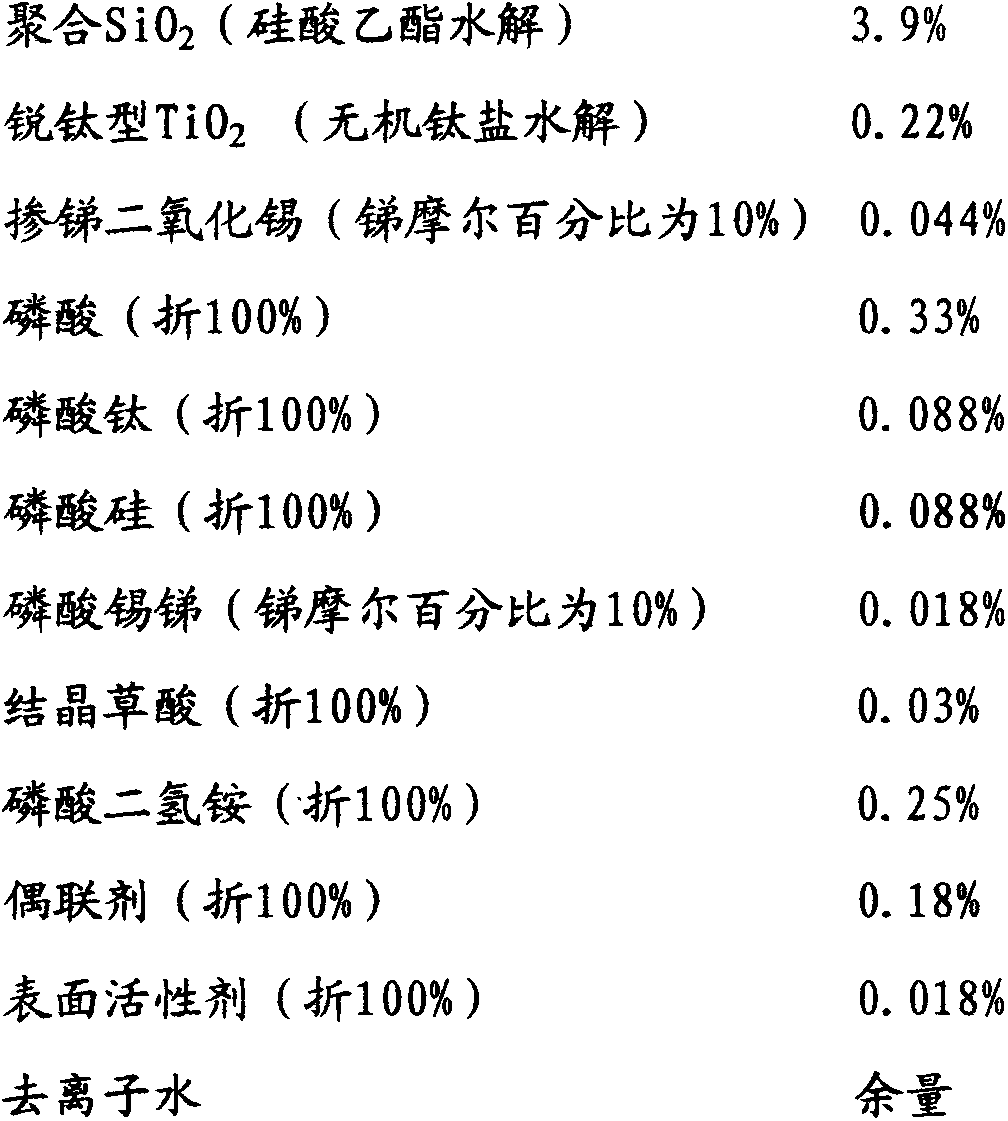
[0046] To a 2000mL four-port reactor equipped with a mechanical stirrer, a thermometer, a dropping funnel, and a condenser, successively add 700 g of ethanol with a mass percentage concentration of 95%, 2.0 g of ammonia water with a mass percentage concentration of 25%, and deionized water. 130g and 160g of orthosilicate ethyl ester with a concentration of 99% by mass, stirred and reacted at 15-30°C for 4-6 hours, left to stand and aged for more than 12 hours to form transparent polymerized SiO 2 Ethanol sol A, the measured average particle size is about 10nm; add 700g of deionized water, and distill 700g of 95% ethanol aqueous solution at 70-75°C to obtain polymerized SiO 2 Aqueous sol B, the measured average particle size is about 10nm; add 30g of dilute phosphoric acid solution with a mass percentage concentration of 20% to adjust the sol to PH2-3 to obtain polymerized SiO 2 Hydrosol C; add anatase nano-TiO with a mass percentage concentration of 5% under stirring 2 300g o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com