Sulfide functionalized covalent organic frame material and synthesis method thereof
A technology of covalent organic framework and synthesis method, applied in chemical instruments and methods, adsorption water/sewage treatment, water/sewage treatment, etc. The effect of high yield and good selective recognition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
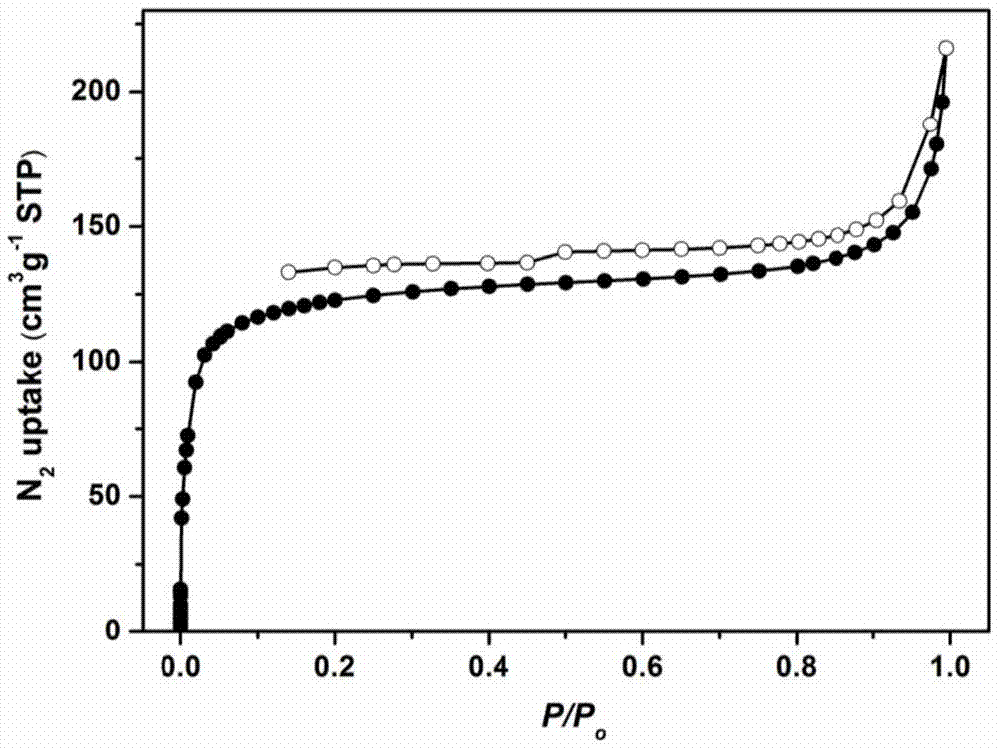
Image
Examples
Embodiment 1
[0023] Add trimesaldehyde (32 mg, 0.20 mmol) and 2,5-bis(3-(ethylsulfide)propyl)-terephthalic hydrazide (130 mg, 0.30 mmol, compound 1) to 1, In the mixed solvent of 4-dioxane (1.0mL) and mesitylene (1.5mL), a white turbid solution was obtained, and 0.4mL of 6.0mol / L aqueous acetic acid solution was slowly added to the above mixed solution to obtain a white The turbid mixture, seal the tube, and then place it in an oven at 120°C for 3 days. After the reaction, the precipitate is washed with acetone (3×10mL), centrifuged, and then extracted by Soxhlet (with tetrahydrofuran, THF). After further purification and drying in a vacuum oven for 8 h, 122 mg of a thioether-functionalized covalent organic framework material (represented by COF-LZU8) was obtained with a yield of 80%.
[0024] The response is as follows:
[0025]
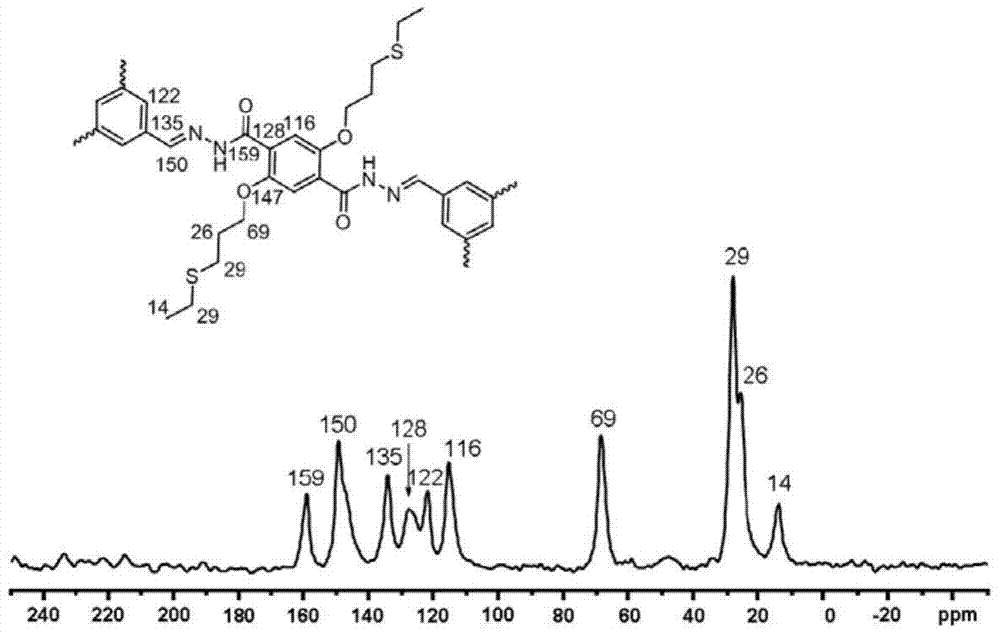
[0026] figure 1 Solid State NMR for COF-LZU8 13 C CP / MAS NMR spectrum.
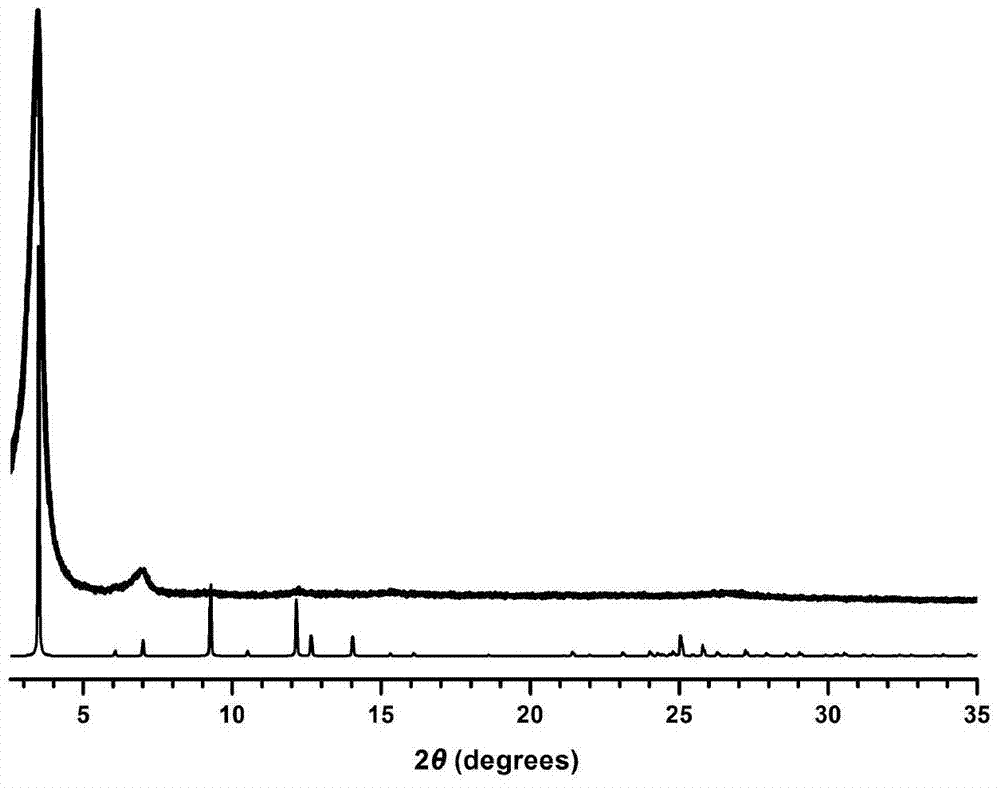
[0027] figure 2 It is the X-ray powder diffraction pattern (PXRD) of COF-LZU8,...
Embodiment 2
[0031] Synthesis of compound 2,5-bis(3-(ethylthioether)propyl)-terephthalohydrazide【2,5-Bis(3-(ethylthio)propoxy)terephthalohydrazide】
[0032] Dissolve ethyl 2,5-dihydroxyterephthalate (2.0 g, 7.87 mmol), bromopropene (4 mL, 47.20 mmol), potassium carbonate (9.0 g), potassium iodide (160 mg) in 30 mL of dry acetone, and Placed in an oil bath and heated to reflux for reaction. After the reaction was monitored by TLC, the acetone was distilled off under reduced pressure and extracted with dichloromethane (3×40 mL). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and separated by column chromatography (petroleum ether / ethyl acetate: 10 / 1, volume ratio) after vacuum distillation to obtain 2.47 g of 2,5-dipropyleneoxy Ethyl phthalate, yield: 95%. 1 H NMR (400MHz, CDCl 3 ):δ=7.38(s,2H),6.10-6.01(m,2H),5.51-5.45(m,2H),5.29-5.27(m,2H),4.60-4.58(m,4H),4.41-4.34 (m,4H),1.38(t,J=8.0Hz,6H). 13 C NMR (100MHz, CDCl 3 ):δ=165.6, 151....
Embodiment 3
[0035] Embodiment 3COF-LZU8 Selective recognition of mercury ions
[0036] The standard solution containing mercury ions was added dropwise to the solution containing COF-LZU8. It can be seen that the fluorescence intensity of COF-LZU8 will gradually weaken with the addition of mercury ions ( Figure 4 ). Under the same test conditions, the influence of other metal ions on the fluorescence intensity of COF-LZU8 solution is very small, far less than that of mercury ions (see Figure 5 ), indicating that COF-LZU8 has a good selective recognition of mercury ions. The same experiment was done with other existing COF materials, and no recognition effect on mercury ions was found.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com