Meloxicam sustained release tablet used for pet and preparation method thereof
A technology of meloxicam and sustained-release tablets, which is applied in the field of meloxicam sustained-release tablets for pets and its preparation, can solve the problems of large influence on the therapeutic effect, excessive fluctuation of blood drug concentration, and dissolution rate of meloxicam. Low-level problems, to achieve the effect of improving clinical treatment effect, reducing the risk of injury, and easy to expand production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
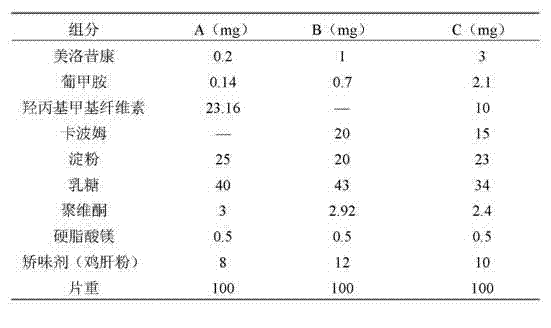
[0021] Party:
[0022]
[0023] Preparation:
[0024] A, firstly prepare the solid dispersion of meloxicam and meglumine, dissolve meloxicam and meglumine in water together, carry out vacuum drying, pulverize the dried solid powder, pass through a 200 mesh sieve, spare;
[0025] B, the meloxicam solid dispersion after sieving, sustained and controlled release auxiliary material, filler, 80% flavoring agent and 30% binding agent mix homogeneously;
[0026] C. Add the ethanol / water (30 / 70) solution of the remaining 70% of the adhesive and mix evenly to make a soft material;
[0027] D. Carry out wet granulation of the prepared soft material, dry at 60°C for 1 hour, and granulate;
[0028] E, add lubricant and remaining 20% correctives to the prepared granule and mix evenly;
[0029] F, tablet pressing, finished product.
Embodiment 2
[0030] Embodiment 2 stability test
[0031] 1 Materials and methods
[0032] 1.1 Material test drugs: meloxicam sustained-release tablets prepared according to Example 1-A, Example 1-B, and Example 1-C respectively; control drugs: according to the patent (CN102525974A, issued on July 4, 2012 Published in Japan) self-made meloxicam tablets with a content of 0.2mg.
[0033] 1.2 Methods Take 180 tablets of the test drug and the control drug respectively. According to the "Technical Specifications for Stability Test of Veterinary Drugs (Trial)", the accelerated test of the drug was carried out for six months. Sampling for content determination.
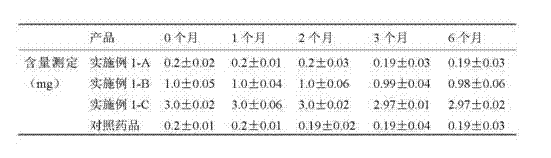
[0034] 2 Test results
[0035] Example 2 Drug Stability Test Results (n=4)
[0036]
[0037] The results show that the drug stability of the meloxicam sustained-release tablets prepared in Example 1-A, Example 1-B, and Example 1-C is obviously better than that of the reference drug. It shows that the preparation is stable.
Embodiment 3
[0038] Example 3 Clinical Trials
[0039] 1. Materials and Methods
[0040] 1.1 Test material
[0041] 1.1.1 Test drug Test drug: Example 1-C, used continuously for seven days; control drug: self-made meloxicam tablets according to the patent (CN102525974A, published on July 4, 2012), with a content of 5 mg.
[0042] 1.1.2 Experimental animals Tianjin Pet Hospital has diagnosed dogs with osteoarthritis, 80 dogs have been diagnosed with the disease, and the age of onset is 9-13 years old.
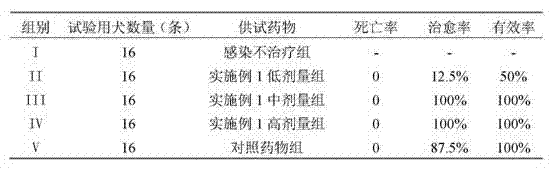
[0043] 1.2 Test method: Divide 80 naturally occurring dogs into five groups of I, II, III, IV and V, with 16 dogs in each group. Group I is the infection-free group; Group II is the group treated with low-dose test drugs (0.1 mg orally administered per kilogram of body weight for the first time, 0.05 mg for the second dose, once every two days, for 5 consecutive times); Group III is the treatment group with a medium dose of the test drug (0.2 mg orally administered for the first time per k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com