High refractive resin containing silicon and preparation method of resin
A technology of resin and hydroxy silicone oil, which is applied in the field of silicon-containing high-refractive resin and its preparation, can solve problems such as poor performance, wear resistance, solvent resistance and poor moisture absorption resistance, and achieve good bonding performance and heat resistance. The effect of high refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
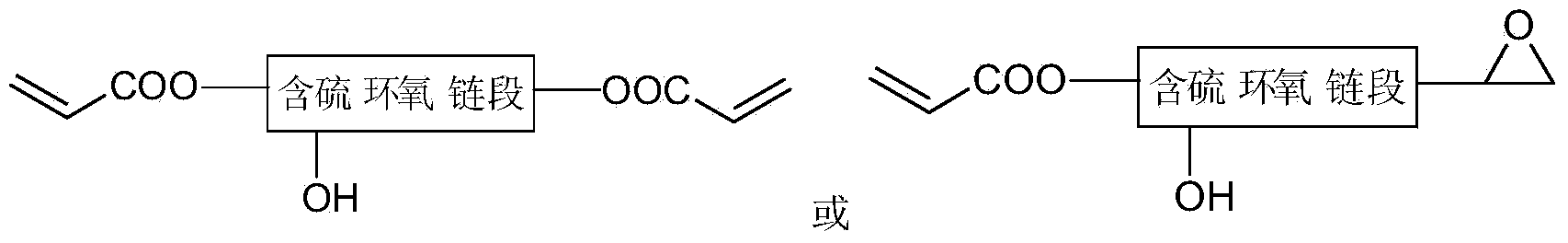
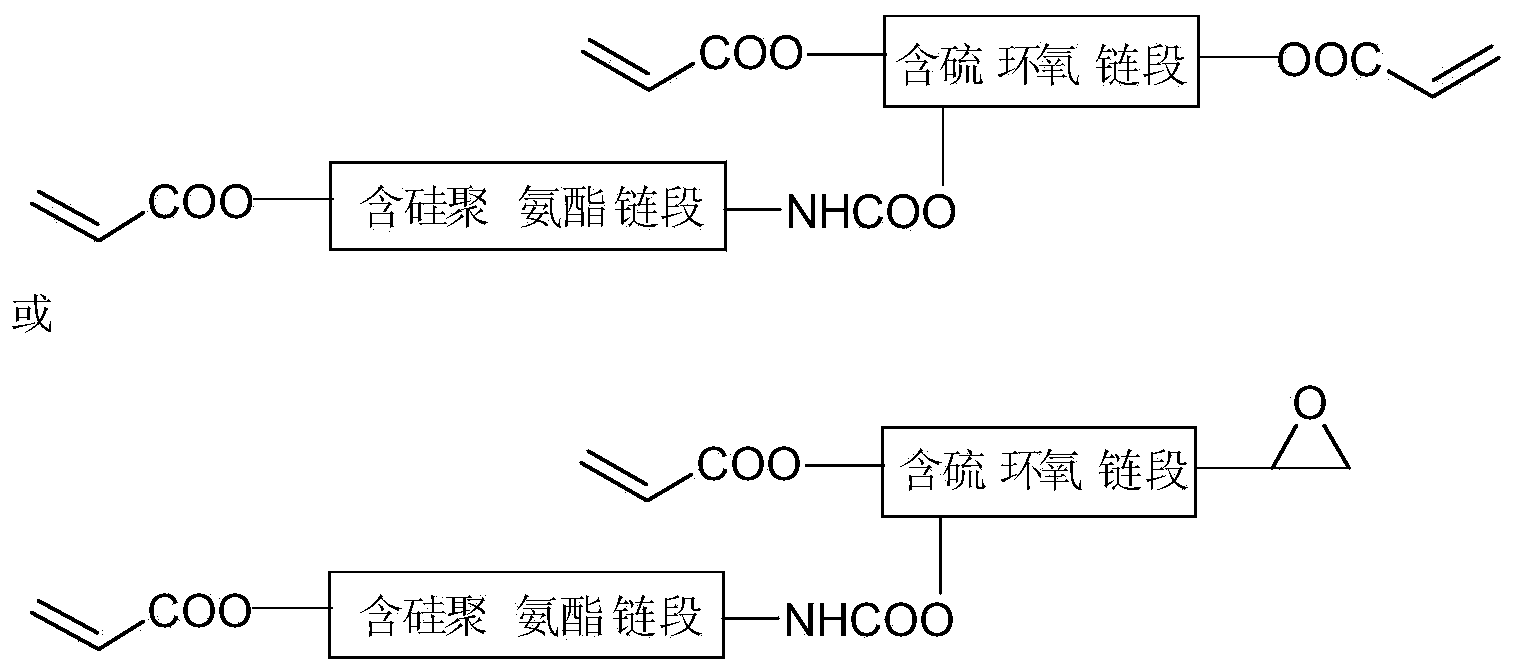
[0026] In the first step, add 3mol of epichlorohydrin into a 1000mL three-necked flask, stir and heat to 65°C, then add 0.6mol of 1,8-octanedithiol, then add about 300mL of 20% NaOH solution, and stir for 1h , cooled to room temperature, added 5 times the volume of diethyl ether and 5 times the volume of distilled water, separated the organic layer, washed to neutrality, dried with anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure to obtain sulfur-containing epoxy; Add 0.4mol of the sulfur-containing epoxy synthesized above into a 500mL three-necked flask, stir and heat to 120°C, then add 0.5% of the catalyst triethylbenzyl ammonium chloride and 1.0% of the polymerization inhibitor p-hydroxybenzyl ether; then add 0.8mol of acrylic acid, and continue to react for 3.5h to obtain sulfur-containing epoxy acrylate ①; in the second step, add 2.0mol of toluene diisocyanate (TDI) into a 1000mL three-necked flask, heat to 65°C, and Add 0.8% of bu...
Embodiment 2
[0028]In the first step, add 2 mol of epichlorohydrin into a 1000 mL three-necked flask, stir and heat to 75°C, then add 0.3 mol of 2,4-dimercaptopyrimidine, then add about 200 mL of 20% NaOH solution, and stir for 1 h. Cool to room temperature, add 3 times the volume of diethyl ether and 4 times the volume of distilled water, separate the organic layer, wash with water until neutral, dry with anhydrous magnesium sulfate, filter, and remove the solvent under reduced pressure to obtain sulfur-containing epoxy; Weigh 0.2 Add the sulfur-containing epoxy synthesized above into a 250mL three-necked flask, stir and heat to 110°C, then add 1.0% of the catalyst tris(acetylacetonate) complex cobalt (Ⅲ) and 0.8% of the polymerization inhibitor p-hydroxybenzene Methyl ether; add the methacrylic acid of 0.4mol again, continue reaction 4.5h, obtain sulfur-containing epoxy acrylate 1.; second step, in the there-necked flask of 500mL, add the diphenylmethane diisocyanate (MDI) of 1.0mol, Hea...
Embodiment 3
[0030] In the first step, add 3mol of epichlorohydrin into a 1000mL three-necked flask, stir and heat to 50°C, then add 0.3mol of 3,6-dioxo-1,8-octanedithiol, and then add about 300mL of 20 %NaOH solution, stirred and reacted for 2h, cooled to room temperature, added 2 times the volume of diethyl ether and 2 times the volume of distilled water, separated the organic layer, washed with water until neutral, dried with anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure to obtain Sulfur-containing epoxy; Weigh 0.2mol of the above-synthesized sulfur-containing epoxy into a 250mL three-necked flask, stir and heat to 100°C, then add 0.8% catalyst N,N-dimethylbenzylamine and 0.5% Inhibitor p-hydroxyanisole; add 0.4mol of methacrylic acid and continue to react for 5h to obtain sulfur-containing epoxy acrylate ①; in the second step, add 1.0mol of hexamethylene in a 500mL three-necked flask Diisocyanate (HDI), heated to 70°C, dibutyltin dilaurate is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com