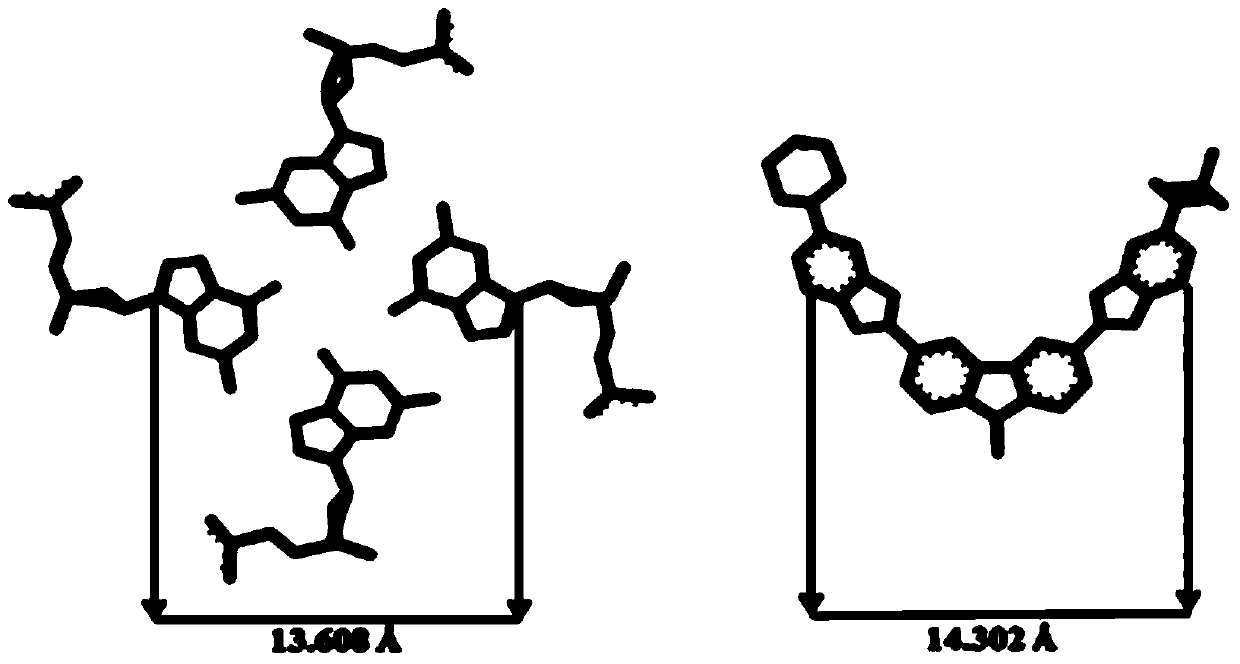
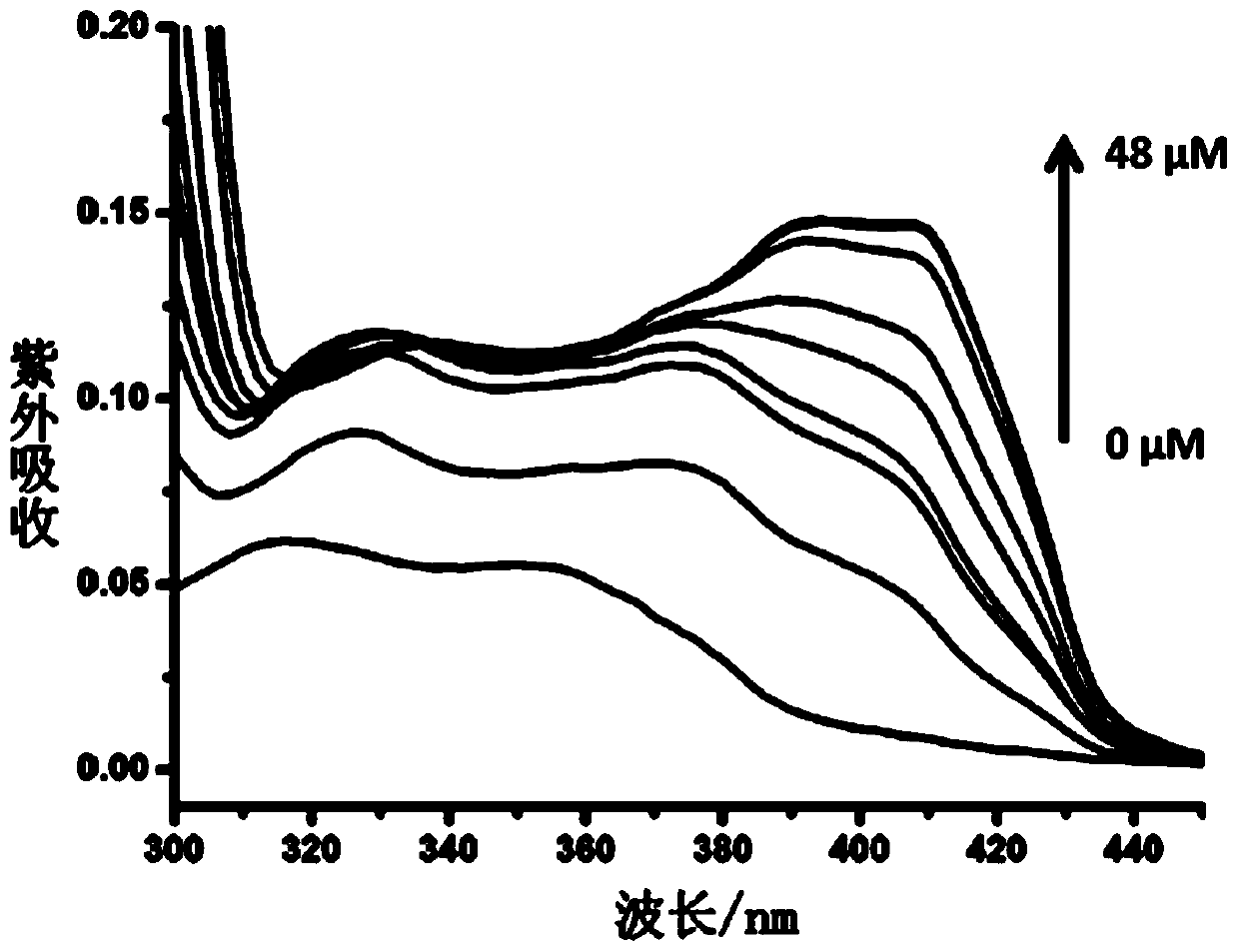
Applications of bisbenzimidazole bicarbazole compound in specific binding nucleic acid G-quadruplex structure and anti-tumor drug
A technology of dibenzimidazole and bicarbazole, which is applied in the application field of anti-tumor drugs and can solve the problems of no change in optical properties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of compound E1:
[0061] (1) Dissolve 1-methylpiperazine (690mg, 6.9mmol) with 5-chloro-2-nitroaniline (1g, 5.8mmol) and potassium carbonate (1.25g, 9mmol) in dry DMF (6mL) , react at a temperature of 110°C for 6 hours to obtain a crude product, place the obtained crude product in water, suspend the crude product in water, extract with ethyl acetate, wash the obtained organic phase with water three times, and then use anhydrous MgSO 4 Drying; purification by neutral alumina column chromatography with chloroform as eluent to obtain 1.1 g of a bright yellow solid, which is compound A1, with a yield of 81%. 1 H NMR (500MHz, CDCl 3 )δ8.01(d,J=9.7Hz,1H),6.28(dd,J=9.7,2.7Hz,1H),6.14(s,2H),5.95(d,J=2.6Hz,1H),3.49- 3.25 (m, 4H), 2.61-2.42 (m, 4H), 2.34 (s, 3H). The structure of the compound A1 is:
[0062]
[0063] The obtained compound A1 was dissolved in ethanol, and the Pd / C with a mass content of 10% was added as a catalyst, and H 2 , r...
Embodiment 2
[0071] Embodiment 2: the synthesis of compound E2:
[0072] (1) Step (1) is basically the same as in Example 1, except that the compound 1-(2-hydroxyethyl)piperazine is used instead of 1-methylpiperazine, and reacted at 140°C overnight to obtain a bright yellow solid compound A2. Yield: 90%; 1 H NMR (500MHz, CDCl 3 )δ8.02(d,J=9.3,1H),6.29(dd,J=9.3,J=2.7,1H),6.1(bs,2H,NH2),5.95(d,J=2.7Hz,1H), 3.8(t,4H), 3.7(t,2H), 2.59-2.65(m,6H). The structure of the compound A2 is:
[0073]
[0074] The obtained compound A2 was dissolved in methanol, and the Pd / C with a mass content of 5% was added as a catalyst, and H 2 , reacted overnight to obtain a compound B2 solution, and filtered out impurities with diatomaceous earth. Since the structure of o-phenylenediamine is easily oxidized and unstable, it is directly put into the next step without purification; the structure of the compound B2 is:
[0075]
[0076] (2) Step (2) was basically the same as in Example 1, except that th...
Embodiment 3
[0082] Embodiment 3: the synthesis of compound E3:
[0083] (1) Step (1) was basically the same as in Example 1, except that the compound morpholine was used instead of 1-methylpiperazine, and reacted at 130° C. for 8 hours to obtain compound A3 as a bright yellow solid. Yield: 75%; 1 H NMR (500MHz, CDCl 3 )δ7.93(d,J=8.4Hz,1H),6.83(bs,J=8,2H),6.18(dd,J=8.4,1.9Hz1H),6.10(d,J=8.8Hz,1H), 3.50-4.00 (m, 4H), 2.40-2.50 (m, 4H). The structure of the compound A3 is:
[0084]
[0085] The obtained compound A3 was dissolved in ethanol, and the Pd / C with a mass content of 5% was added as a catalyst, and H 2 , reacted overnight to obtain a compound B3 solution, and filtered out impurities with diatomaceous earth. Since the structure of o-phenylenediamine is easily oxidized and unstable, it is directly put into the next step without purification; the structure of the compound B3 is:
[0086]
[0087] (2) Step (2) was basically the same as in Example 1, except that the compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com