Furanone ether derivative and preparation method thereof, and application of furanone ether derivative in antibacterial agent
A technology of furanone ethers and derivatives, applied in antibacterial drugs, organic chemistry, etc., can solve problems such as loss of life, and achieve the effects of high activity, good transmission in the body, and good inhibition and killing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
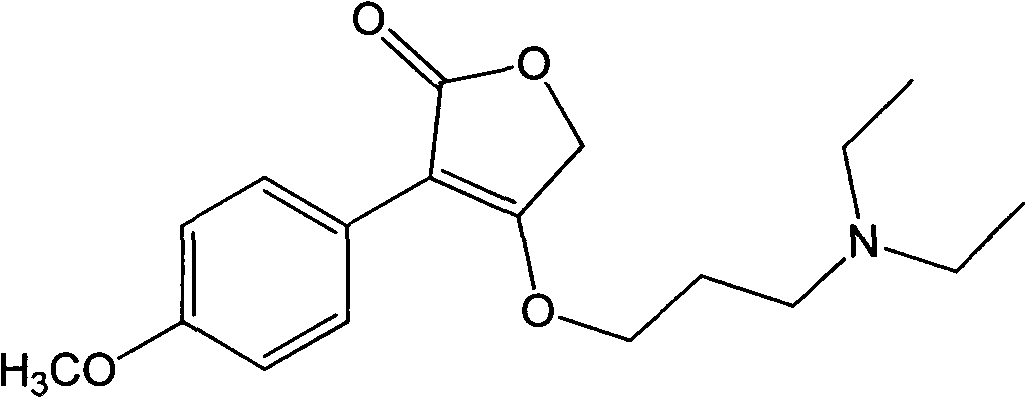
[0019] Example 1: Preparation of 4-(3-(diethylamine)propoxy)-3-(4-methoxyphenyl)furan-2(5H)-one (Compound 1)
[0020]
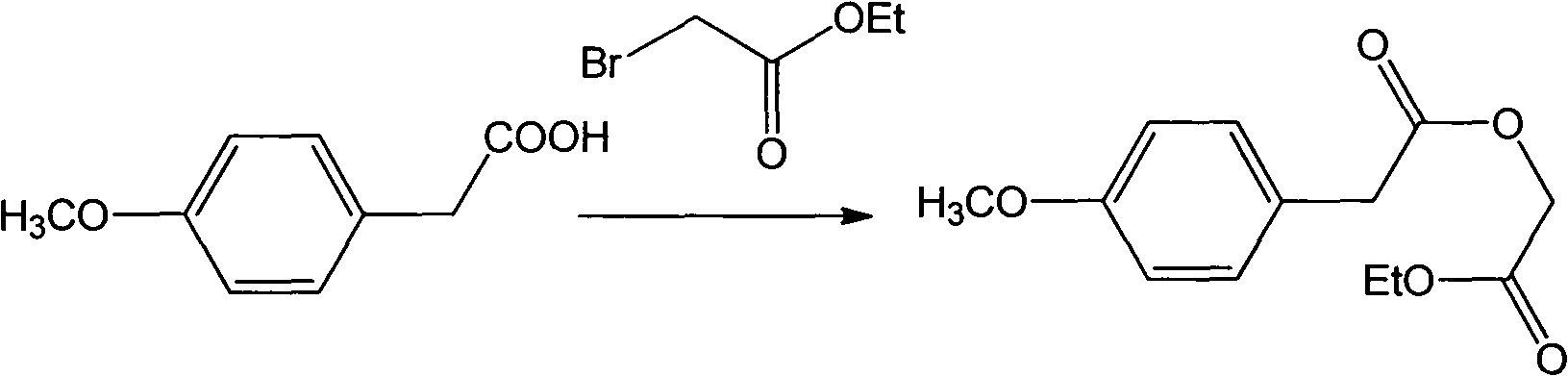
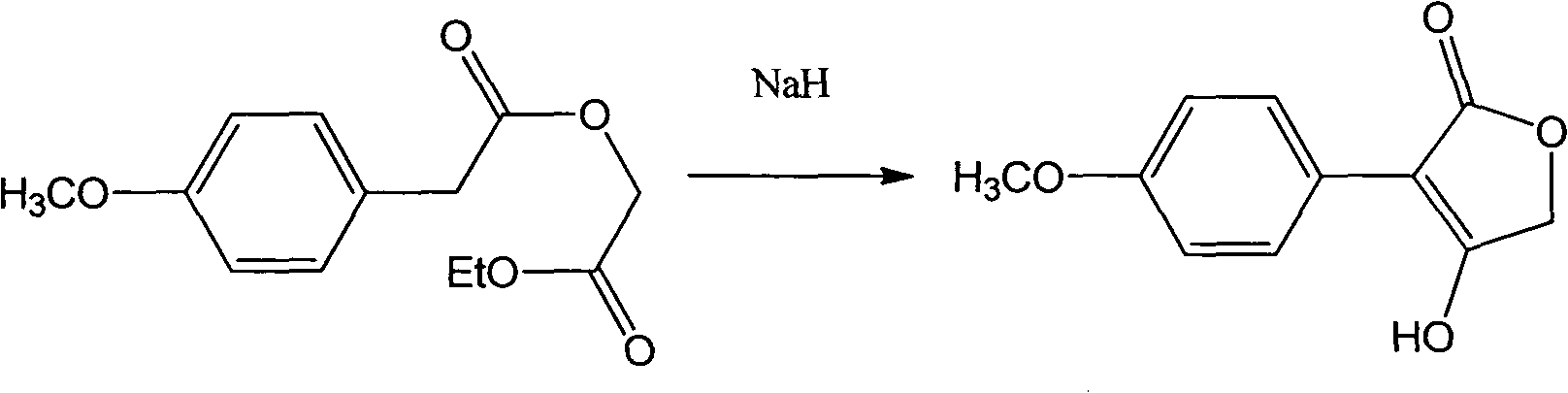
[0021] Dissolve 5mmol of p-methoxyphenylacetic acid and 15mmol of sodium ethylate in 50mL of absolute ethanol, add 10mmol of ethyl bromoacetate at room temperature, raise the temperature to 45°C and react for 10h (detected by TLC). After the reaction, filter with suction and concentrate to One-tenth of the original volume, diluted with 50mL ether, washed 3 times with 30mL water (10mL each time), washed the organic layer with saturated brine until neutral, dried, concentrated, separated by silica gel column chromatography, and the eluent was petroleum ether The mixed solution with a volume ratio of 10:1 to ethyl acetate was concentrated; the anhydrous tetrahydrofuran solution containing 3 mmol of the obtained product was dropped into anhydrous tetrahydrofuran containing 3 mmol of sodium hydride at room temperature, and the dropwise reaction was completed at ...
Embodiment 2
[0022] Embodiment two: the antibacterial activity of compound
[0023] Bacteria were suspended in MH medium at a concentration of approximately 10 5 cfu / mL, the bacterial solution was added to a 96-well plate (100 μL of bacterial solution per well), the culture medium was used as a blank control, DMSO was used as a negative control instead of the test substance, and kanamycin was used as a positive control. Dissolve the test substance in DMSO to make 1600, 800, 400, 200, 100, 50 μg / mL solutions respectively (for MIC 50 If it is less than 5 μg / mL, in further experiments, the prepared concentration gradient is 100, 50, 25, 12.5, 6.25 μg / mL), and added to the 96-well plate in an amount of 11 μL per well [the final concentration of the drug solution is 160 , 80, 40, 20, 10, 5 μg / mL (10, 5, 2.5, 1.25, 0.63 μg / mL for the latter)], four parallel experiments were done for each concentration gradient. Place the 96-well plate in an incubator at 37°C for 24 hours, then add 25 μL per we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com