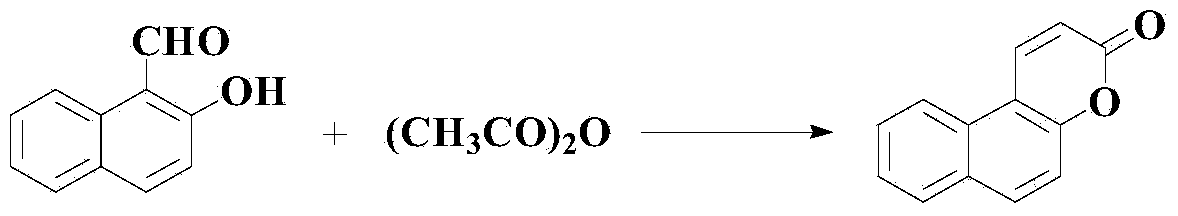Improved synthetic method of aryl substituted naphthopyran photochromic compound
A kind of technology of naphthopyrans, synthetic method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0033] Preparation Example 1: Synthesis of Naphthopyran-2-one
[0034] Add 1mol of 2-hydroxy-1-naphthaldehyde, 2.2mol of anhydrous acetic anhydride and 2.1mol of anhydrous sodium acetate in the reaction kettle, heat up to reflux for 5 hours, after the reaction is completed, cool the reaction solution to room temperature, add Aqueous sodium carbonate solution with a mass concentration of 12% adjusted the pH to neutral to remove unreacted acetic anhydride, then added chloroform to dissolve the solid product, separated the liquid and dried with anhydrous magnesium sulfate, filtered and rotary evaporated, then added petroleum ether Promote the formation of precipitates, filter again, and dry to obtain the intermediate naphthopyran-2-one with a yield of 90% and a purity of 94.7% as detected by high performance liquid chromatography.
Embodiment 1
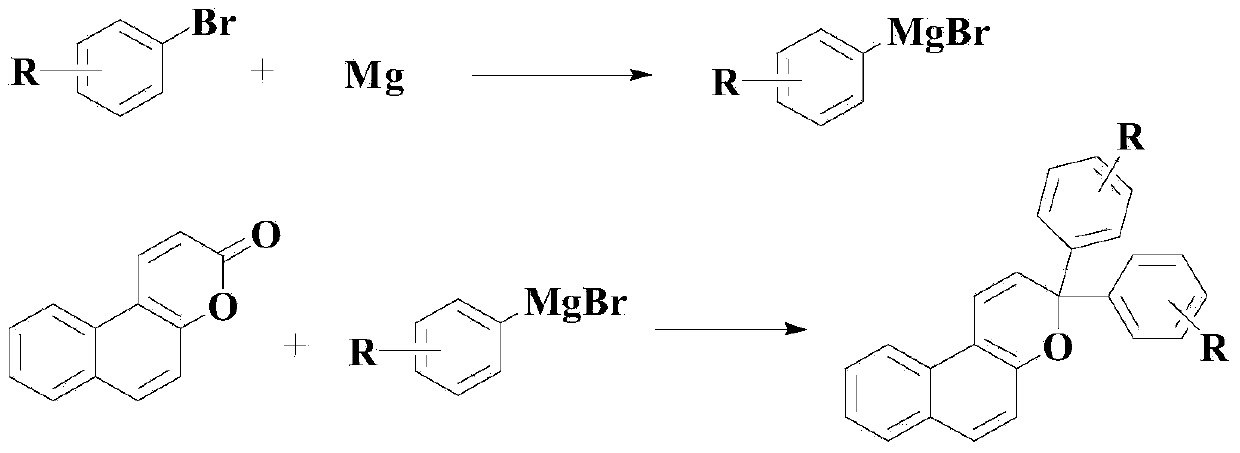
[0035] Example 1: Synthesis of 2,2-bis(3-methylphenyl)naphthopyran
[0036] Under a nitrogen atmosphere, add 0.6mol of magnesium chips to the reaction kettle, then dropwise add a mixture containing 1.2mol of 3-methyl-1-bromobenzene and 50ml of THF, gradually raise the temperature to reflux reaction, and react until the reaction of magnesium chips is complete, stop Reaction to obtain the Grignard reagent; in the prepared Grignard reagent, add 0.2mol of naphthopyran-2-one, add 0.02mol of tetrabutylphosphine bromide and 180ml of 1-butyl-3- Methylimidazolium chloride salt, heated at 60°C and kept for 7 hours. After the reaction, add ammonium chloride aqueous solution with a mass concentration of 22% for hydrolysis, then add ether for extraction, dry with anhydrous magnesium sulfate, and undergo recrystallization and column chromatography. Purification gave the target product with a yield of 85.2% and a purity of 98.7% (HPLC).
Embodiment 2
[0037] Example 2: Synthesis of 2,2-bis(3-trifluoromethylphenyl)naphthopyran
[0038] Under a nitrogen atmosphere, add 0.8mol magnesium chips to the reaction kettle, then dropwise add a mixed solution containing 1.6mol 3-trifluoromethyl-1-bromobenzene and 65ml THF, gradually raise the temperature to reflux reaction, and react until the magnesium chips are completely reacted , stop the reaction to obtain the Grignard reagent; in the prepared Grignard reagent, add 0.25mol of naphthopyran-2-one, add 0.015mol of tetrabutylphosphine bromide and 250ml of 1-butyl- 3-Methylimidazolium chloride, heated at 70°C and kept for 6.5 hours. After the reaction, add ammonium chloride aqueous solution with a mass concentration of 22% for hydrolysis, then add ether for extraction, dry with anhydrous magnesium sulfate, recrystallize and Purified by column chromatography to obtain the target product with a yield of 86.3% and a purity of 98.9% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com