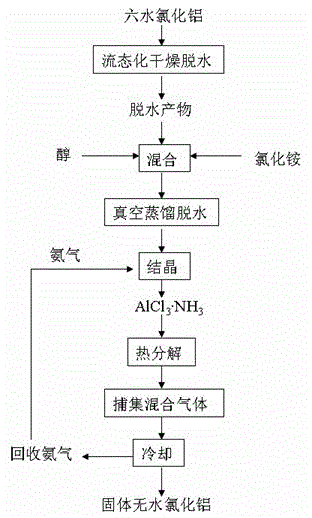
A method for preparing anhydrous aluminum chloride by dehydration of aluminum chloride hexahydrate
A technology of aluminum chloride hexahydrate and anhydrous aluminum chloride, which is applied in the direction of aluminum chloride and aluminum halide, can solve the problems of complex process flow, low purity of anhydrous aluminum chloride, and difficult operation, and achieves wide sources, The effect of wide range of process conditions and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Take 400 grams of aluminum chloride hexahydrate, dry and dehydrate at 150 ° C, the weight after drying is 246 grams, and the weight loss is 154 grams. Aluminum chloride hexahydrate is dried at 150°C, partial hydrolysis occurs, and AlCl in the dried product 3 ·H 2 O accounted for 68.2%.
Embodiment 2
[0043]Dry aluminum chloride hexahydrate at 150°C and store in sealed condition. Take 150 grams of dried aluminum chloride powder and dissolve it in 1000 ml of ethylene glycol solvent, add 35 grams of ammonium chloride at the same time, and adopt the Karl Fischer method to measure the water content of the solution to be 2.45%. Place the prepared solution in a 2000ml three-necked distillation flask, perform intermittent vacuum distillation and dehydration operation for 180min at a vacuum degree of 650mmHg and a temperature of 150°C, and measure the water content of the solution after vacuum distillation and dehydration to be 0.87%. Take out the ethylene glycol solution of 800ml aluminum chloride from the solution after distillation and dehydration and cool it, add 100ml liquid ammonia, then feed ammonia gas, and generate AlCl in the fluidized bed reaction crystallizer 3 ·NH 3 The precipitate was filtered, washed three times with ethylene glycol saturated with liquid ammonia, an...
Embodiment 3
[0045] Take 100 grams of aluminum chloride hexahydrate, without drying, directly dissolve in 1000ml of ethylene glycol, and use the Karl Fischer method to measure the water content of the solution to be 6.8%. The ethanol solution of aluminum chloride of configuration does not go through the step of vacuum distillation dehydration, directly carries out reaction crystallization process (reaction crystallization, filtration, washing and drying process) and example 2 is identical, the AlCl that the result obtains 3 ·NH 3 The crystals are impure, and the analysis shows that AlCl 3 ·NH 3 The content is about 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com