Sulfonate type biomass surfactant and synthesis method thereof
A technology of surfactant and sulfonate type, which is applied in the direction of sulfonate preparation, chemical instruments and methods, and drilling compositions, etc. It can solve the difficulties in purification of reaction products, limited use range, general temperature resistance, etc. problems, to achieve good application prospects, low cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The synthetic method specifically comprises the following steps:
[0033] 1) Preparation of fatty acid alkanolamides
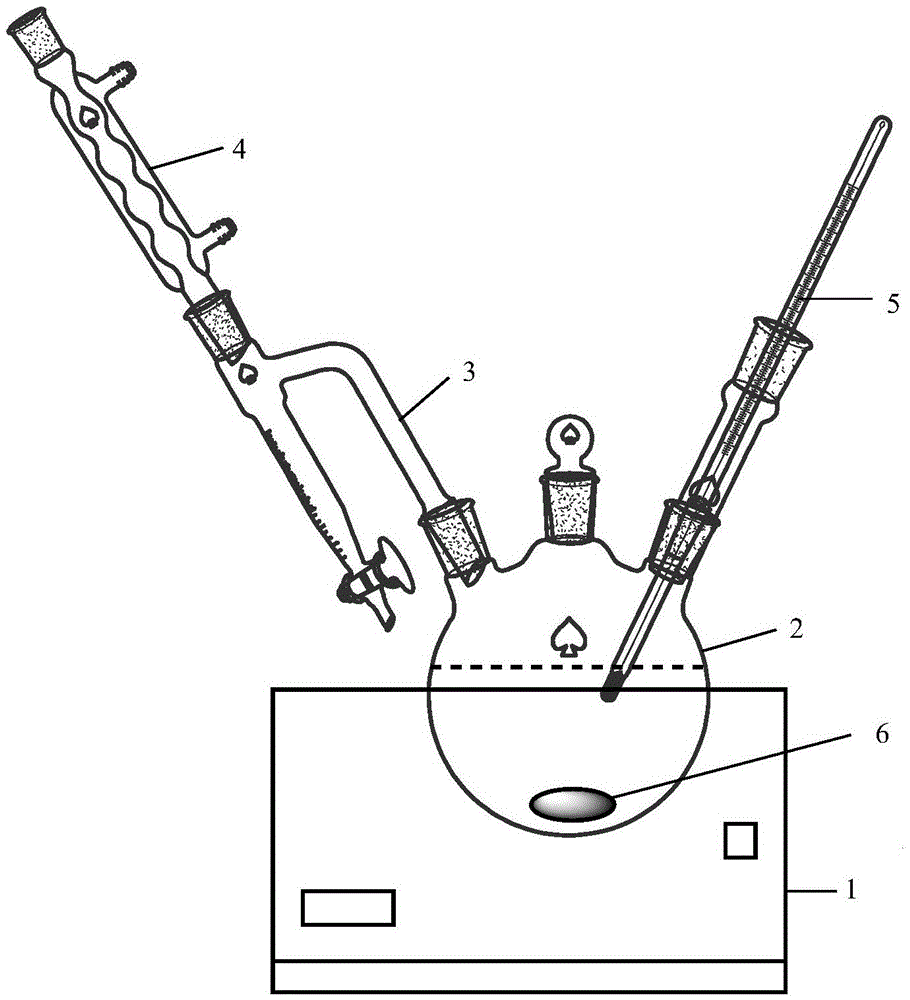
[0034] Mix fatty acid methyl ester and diethanolamine in a molar ratio of 1:1.05 to 1:1.15 in a three-necked flask 2 equipped with a magnetic rotor 6, a condenser tube 4, and a thermometer 5, and add the total reactant (fatty acid methyl ester) into the three-necked flask. The sum of the mass of ester and diethanolamine) catalyst sodium hydroxide with a mass of 0.6% to 0.8%, then put the flask in an oil bath and heat it to 110°C to 130°C to stir and react for 4h to 6h, and finally spin evaporate, dry and cool to obtain fatty acid Alkanolamides.
[0035] The three-neck flask 2 is a glass container commonly used in the synthesis reaction of organic substances. It usually has the appearance of a round belly and a thin neck. It has three mouths, and a water separator 3 and a condenser pipe 4 are installed on one side mouth to condense the evaporated steam ...
Embodiment 1
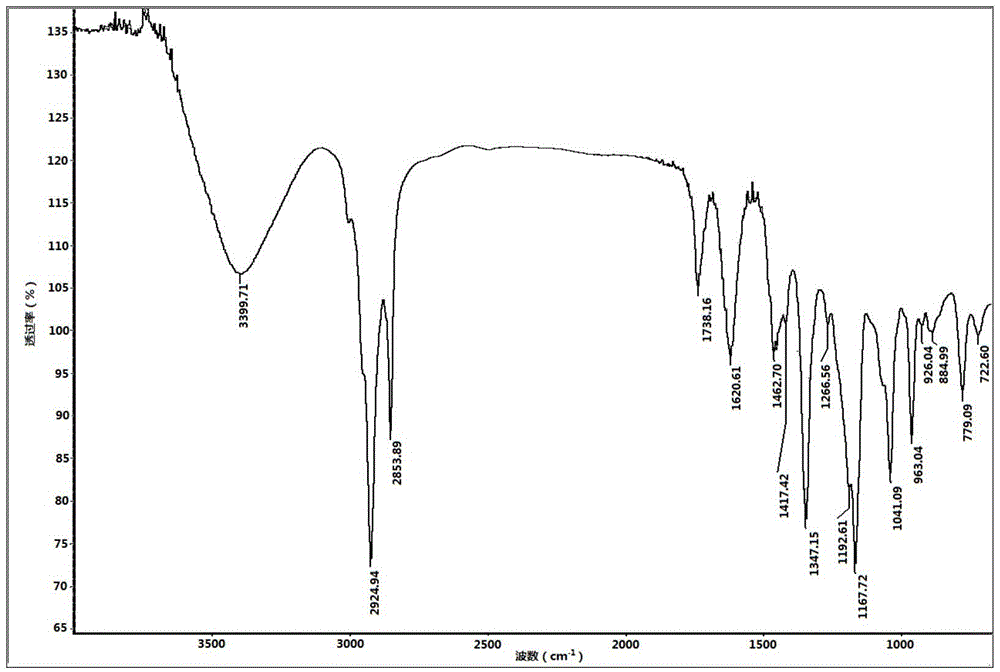
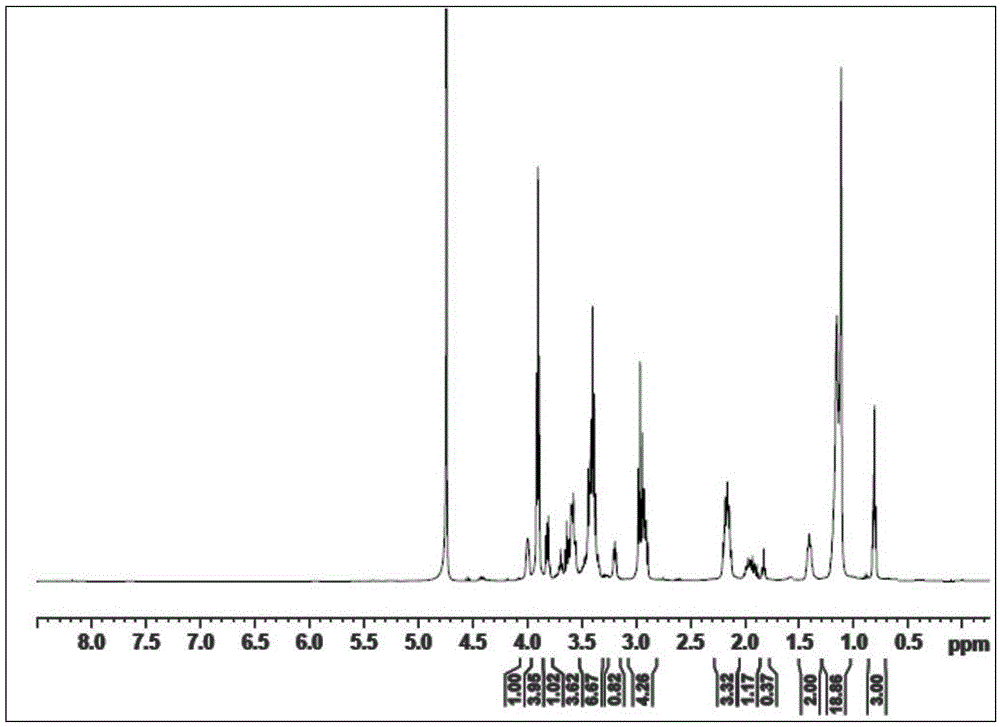
[0041] 21.43g methyl laurate (C 11 h 23 COOCH 3 , 0.1 mole) and 11.04g diethanolamine (0.105 mole) are mixed in the three-necked flask that magnetic rotor, condenser, thermometer are housed, and in the three-necked flask, add 0.20g sodium hydroxide (accounting for the mass percentage of total reactant is 0.62% ), the flask was placed in an oil bath and heated to 110°C, stirred and reacted for 5 hours, and then dried and cooled by rotary evaporation to obtain 24.21g of lauric acid alkanolamide with a yield of 84.24%.
[0042] Dissolve 12.20g of propane sultone (0.1 mol) in a certain volume of tetrahydrofuran, then add 14.66g (0.051 mol) of lauric acid alkanolamide to it, fully dissolve it and put it into a In a three-necked flask, add 0.27g sodium hydroxide (accounting for 1.00% by mass of the total reactant) into the three-necked flask, heat to reflux temperature, stir and react for 16h, and obtain 14.42g N,N-di (1-ethoxy-)3-sulfopropyl lauramide sulfonate surfactant, the y...
Embodiment 2
[0047] 21.43g methyl laurate (C 11 h 23 COOCH 3 , 0.1 mole) and 11.56g diethanolamine (0.11 mole) are mixed in the three-necked flask that magnetic rotor, condenser, thermometer are housed, and in the three-necked flask, add 0.231g sodium hydroxide (accounting for the mass percentage of total reactant is 0.70% ), the flask was placed in an oil bath and heated to 120°C, stirred and reacted for 5 hours, and then dried and cooled by rotary evaporation to obtain 24.55g of lauric acid alkanolamide with a yield of 85.41%.
[0048] Dissolve 12.20g propane sultone (0.1 mole) in a certain volume of tetrahydrofuran, then add 15.09g (0.0525 mole) of lauric acid alkanolamide to it, fully dissolve and put it into the In a three-necked flask, add 0.33g sodium hydroxide (accounting for 1.21% by mass of the total reactant) into the three-necked flask, heat to reflux temperature, stir and react for 12h, and obtain 13.45g N,N-di (1-ethoxy-)3-sulfopropyl lauramide sulfonate surfactant, the yi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| interfacial tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com