Method for precipitation separation and recovery of chromium and vanadium in chromium-vanadium solution
A precipitation separation and solution technology, applied in the direction of process efficiency improvement, etc., can solve the problems of limited extractant capacity, not suitable for transformation, and difficulty in obtaining high-concentration vanadium liquid, and achieve low anti-corrosion requirements, less water consumption, and variety little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
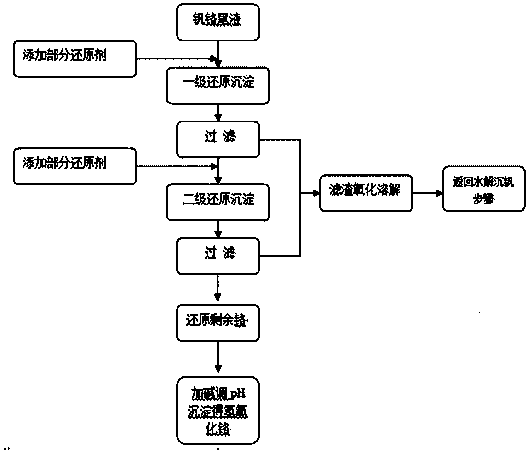
Image
Examples
Embodiment 1
[0025] (1) Take 2L of vanadium-chromium mixed solution, which contains 1050ppm Cr(VI) and 1090ppm V(V), heat it to 80 degrees, adjust the pH value to 3, and add 3 times the theoretical amount of ammonium sulfate to precipitate vanadium;
[0026] (2) Take 1L of vanadium precipitation tail liquid, which contains 1020ppm Cr(VI) and 205ppm V(V);
[0027] (3) Add 0.5g composite reducing agent, adjust pH=2.3 with 1:1 sulfuric acid, stir for 10min; add 3mol / L caustic soda solution to adjust pH, precipitate and stand still;
[0028] (4) Pour out the supernatant and filter the precipitate at the bottom; add 0.25g composite reducing agent after mixing the filtrate and the supernatant, adjust the pH=2.2, stir for 10min, add caustic soda solution to adjust the pH=4.6, precipitate and let stand;
[0029] (5) Pour out the supernatant, filter the precipitate at the bottom; add 23g of composite reducing agent after mixing the filtered liquid and the supernatant, adjust the pH=2.3, stir for 10...
Embodiment 2
[0033] (1) Take 2L vanadium-chromium mixed solution, which contains 1580ppm Cr(VI) and 1255ppm V(V), heat it to 90 degrees, adjust the pH value to 1.6, add 2 times the theoretical amount of ammonium sulfate to precipitate vanadium;
[0034] (2) Take 1L of vanadium precipitation tail liquid, which contains 1500ppm Cr(VI) and 202ppm V(V);
[0035] (3) Slowly inject 1L containing 10% SO 2 The air is circulated through the closed air collection device until it is completely absorbed. Use 1:1 sulfuric acid to adjust pH=2.3, stir for 10 minutes; add 3mol / L caustic soda solution to adjust pH=7.5, precipitate and stand still;
[0036] (4) Pour out the supernatant, filter the precipitate at the bottom; mix the filtered liquid with the supernatant, and then slowly pass through 0.5L containing 10% SO 2 The air is circulated through a closed gas collection device until it is completely absorbed. Adjust pH = 2.2, stir for 10 minutes, add caustic soda solution to adjust pH = 4.6, precipit...
Embodiment 3
[0042] (1) Take 10L vanadium-chromium mixed solution, which contains 1020ppm Cr(VI) and 2010ppm V(V), heat it to 80 degrees, adjust the pH value to 2.1, add 1.5 times the theoretical amount of ammonium sulfate and ammonium nitrate 1:1 Mixed reagent vanadium precipitation;
[0043] (2) Take 5L of vanadium precipitation tail liquid, which contains 990ppm Cr(VI) and 180ppmV(V);
[0044] (3) Add a compound reducing agent that completely reduces the theoretical amount of 5%, adjust the pH=1.5 with 1:1 sulfuric acid, and stir for 60 minutes; add 5mol / L caustic soda solution to adjust the pH, precipitate and stand still;
[0045] (4) Pour out the supernatant, filter the bottom sediment; mix the filtered liquid with the supernatant, add 45% of the theoretical amount of complete reduction compound reducing agent, adjust the pH = 2.2, stir for 60 minutes, add caustic soda solution to adjust the pH = 6, and precipitate and stand still;
[0046] (5) Pour out the supernatant, filter the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com