A method of removing residue hydrogen fluoride in fluosilicic acid
A technology of fluorosilicic acid and hydrogen fluoride, which is applied in the chemical field, can solve the problems of increasing the production cost of lead electrolyte and the extra consumption of lead oxide, and achieve the effects of reducing dilution, reducing the amount of treatment, and saving the amount of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
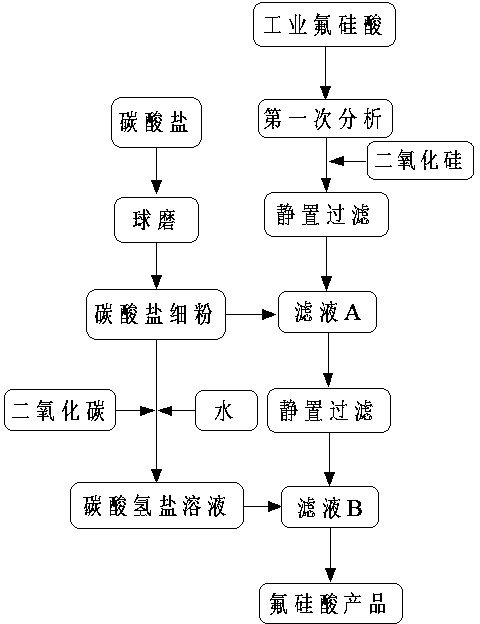
[0037] A method for removing residual hydrogen fluoride in fluosilicic acid, comprising the steps of:
[0038] 1) Using 1 kg of commercially available calcium carbonate, mill it in a ball mill containing agate grinding balls for 60 minutes to obtain calcium carbonate powder;
[0039] 2) Use a 320-mesh sieve to sieve the calcium carbonate powder obtained in step 1), take the fine powder that has been sun-dried, and return the calcium carbonate left on the sieve to the process of step 1) for ball milling. After multiple ball mills, the cumulative A total of 0.96 kg of calcium carbonate was obtained;
[0040] 3) The commercially available 10 liters of fluorosilicic acid with a concentration of 41% by weight was determined by chemical titration analysis, and the density was about 1.2g / L; the titration analysis showed that the content of fluorosilicic acid was 557.2g / L, hydrogen fluorine The acid content is 63.5g / L;
[0041]4) Add 600 grams of silica powder to the industrial fluo...
Embodiment 2
[0043] A method for removing residual hydrogen fluoride in fluosilicic acid, comprising the steps of:
[0044] 1) Using 1 kg of commercially available calcium carbonate, mill it in a ball mill containing agate grinding balls for 60 minutes to obtain calcium carbonate powder;
[0045] 2) Use a 320-mesh sieve to sieve the calcium carbonate powder obtained in step 1), take the fine powder that has been sun-dried, and return the calcium carbonate left on the sieve to the process of step 1) for ball milling. After multiple ball mills, the cumulative A total of 0.96 kg of calcium carbonate was obtained;
[0046] 3) Take 10 liters of industrial fluosilicic acid (such as produced by Haoda Industrial Co., Ltd.), and use the chemical titration method to analyze and measure the sample, showing that the content of fluosilicic acid is 557.8g / L, and the content of hydrofluoric acid is 54.7g / L;
[0047] 4) Add 500 grams of commercially available silica powder to the industrial fluosi...
Embodiment 3
[0053] A method for removing residual hydrogen fluoride in fluosilicic acid, comprising the steps of:
[0054] 1) Using 1 kg of commercially available calcium carbonate, mill it in a ball mill containing agate grinding balls for 60 minutes to obtain calcium carbonate powder;
[0055] 2) Use a 320-mesh sieve to sieve the calcium carbonate powder obtained in step 1), take the fine powder that has been sun-dried, and return the calcium carbonate left on the sieve to the process of step 1) for ball milling. After multiple ball mills, the cumulative A total of 0.96 kg of calcium carbonate was obtained;
[0056] 3) Take 10 liters of commercially available industrial fluosilicic acid, and use the chemical titration method to analyze the sample for the first time, showing that the content of fluosilicic acid is 557.4g / L, and the content of hydrofluoric acid is 55.3g / L;
[0057] 4) Add 500 grams of commercially available silica powder to industrial fluosilicic acid for the firs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com