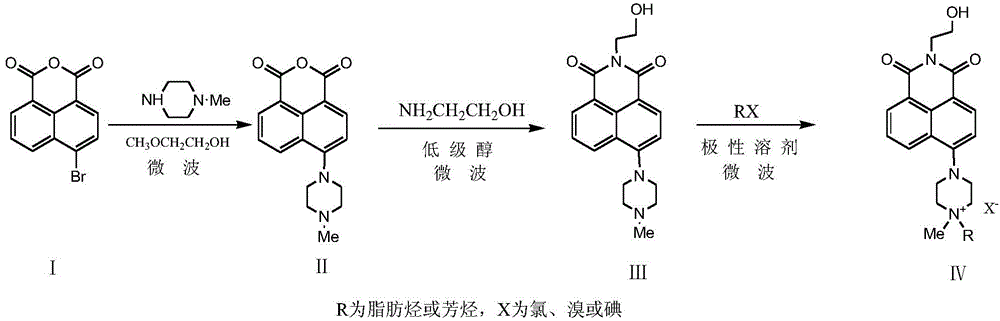
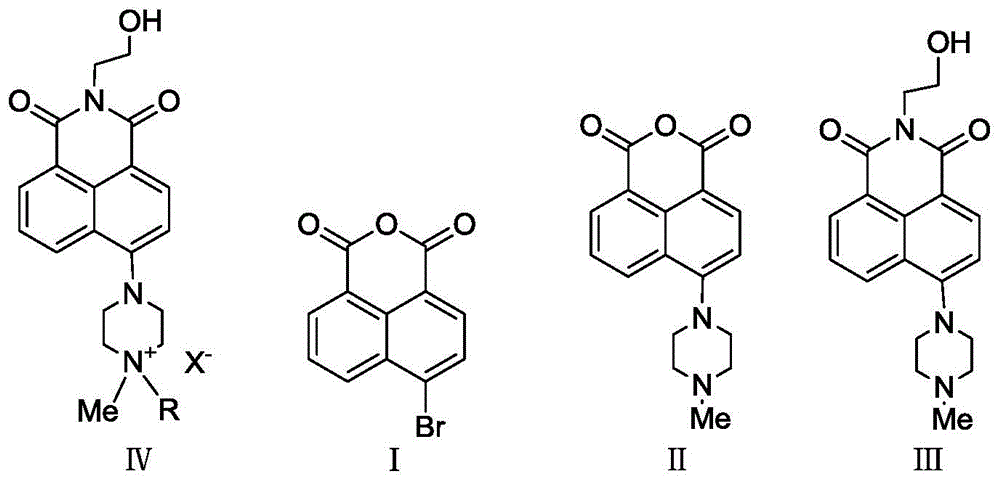
A kind of microwave atmospheric pressure synthetic method of naphthalimide quaternary ammonium salt
A naphthalimide, atmospheric synthesis technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of poor water solubility of naphthalimide, limited application range, long reaction time, etc. Purification, no secondary pollution, less solvent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Synthesis of Compound II: 0.01mol 4-bromo-1,8-naphthalene anhydride, 0.03mol N-methylpiperazine and 10mL ethylene glycol monomethyl ether were added in turn to a 100mL flask, placed in a microwave reactor, 530W Microwave pulse radiation reaction under high power, pulse width is 65s, duty ratio is 12:1, radiation reaction is 65min, and the end point of the reaction is monitored by TLC method (developing agent: dichloromethane: methanol: triethylamine=18:8:0.5) , until R f The raw material point of 0.54 disappears. Add 40 mL of glacial acetic acid to the mixture, freeze to crystallize, filter after thawing, collect the solid, wash with 20 mL of absolute ethanol 2 to 3 times, and dry the solid under vacuum at 40°C / -0.1Mpa to obtain a dark yellow solid with a yield of 97.6%.
[0029] IR (KBr, ν / cm -1 ): 1465.26, 1399.37 (-CH 3 Deformation vibration); 1339.99, 1350.91 (C-N stretching vibration), 2687.92, 2607.71 (multiple frequency of C-N stretching); at the same time...
Embodiment 2
[0047] (1) Synthesis of Compound II: The reaction conditions are the same as step (1) in Example 1, except that the pulsed radiation power is 450W, the radiation reaction time is 70min, and the post-treatment method is the same as step (1) in Example 1. , a dark yellow solid was obtained with a yield of 92.5%.
[0048] (2) Synthesis of Compound III: The reaction conditions are the same as step (2) in Example 1, except that the lower alcohol is methanol, the pulsed radiation power is 250W, and the post-treatment method is the same as step (2) in Example 1. A yellow-green solid was obtained with a yield of 75.7%.
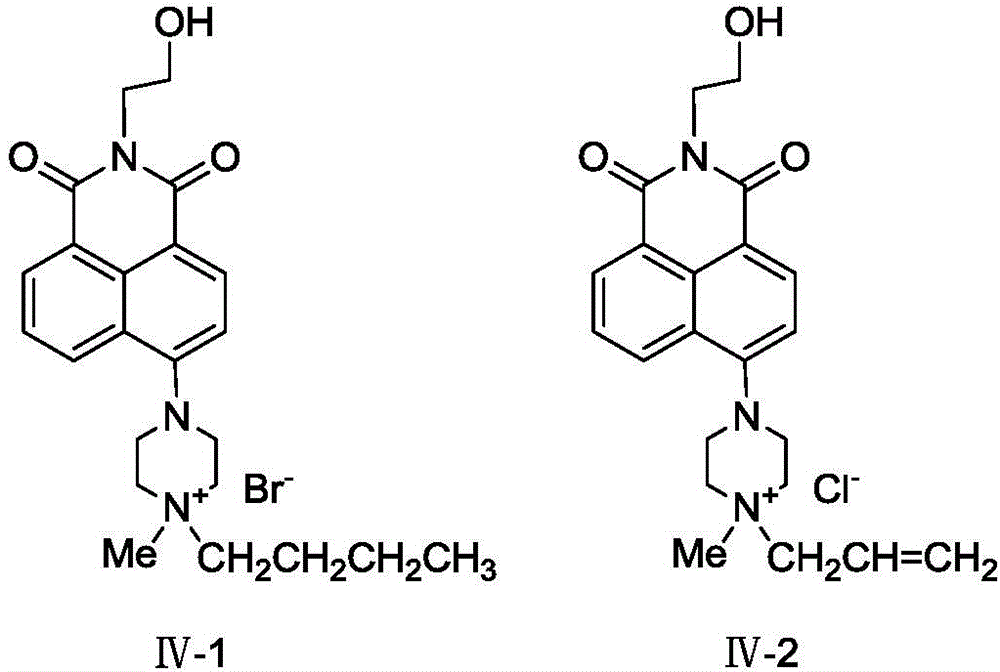
[0049] (3) Synthesis of compound IV-1: the reaction conditions are the same as step (3) in Example 1, the difference is that the pulsed radiation power is 450W, and the post-treatment method is the same as step (3) in Example 1 to obtain bright yellow Solid, 98.3% yield.
Embodiment 3
[0051] (1) Synthesis of Compound II: The reaction conditions are the same as step (1) in Example 1, except that the pulsed radiation power is 600W, the radiation reaction time is 55min, and the post-treatment method is the same as step (1) in Example 1. , a dark yellow solid was obtained with a yield of 93.3%.
[0052] (2) Synthesis of Compound III: The reaction conditions are the same as step (2) in Example 1, except that the pulsed radiation power is 530W, the radiation reaction time is 12min, and the post-treatment method is the same as step (2) in Example 1. , to obtain a yellow-green solid with a yield of 76.4%.
[0053] (3) Synthesis of compound IV-1: The reaction conditions are the same as step (3) in Example 1, except that the molar ratio of raw materials is 1:2 (compound III: 0.005mol, 1-bromobutane: 0.010 mol), the post-treatment method was the same as step (3) in Example 1 to obtain a bright yellow solid with a yield of 80.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com