Cardanol phenolic-group non-ionic type dimeric surfactant and synthetic method thereof
A non-ionic, gemini surface technology, applied in chemical instruments and methods, preparation of aminohydroxy compounds, preparation of organic compounds, etc., can solve the problems of staying in the laboratory stage, high price, difficult synthesis, etc., and achieve the source of raw materials. The effect of low cost, low production cost and short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
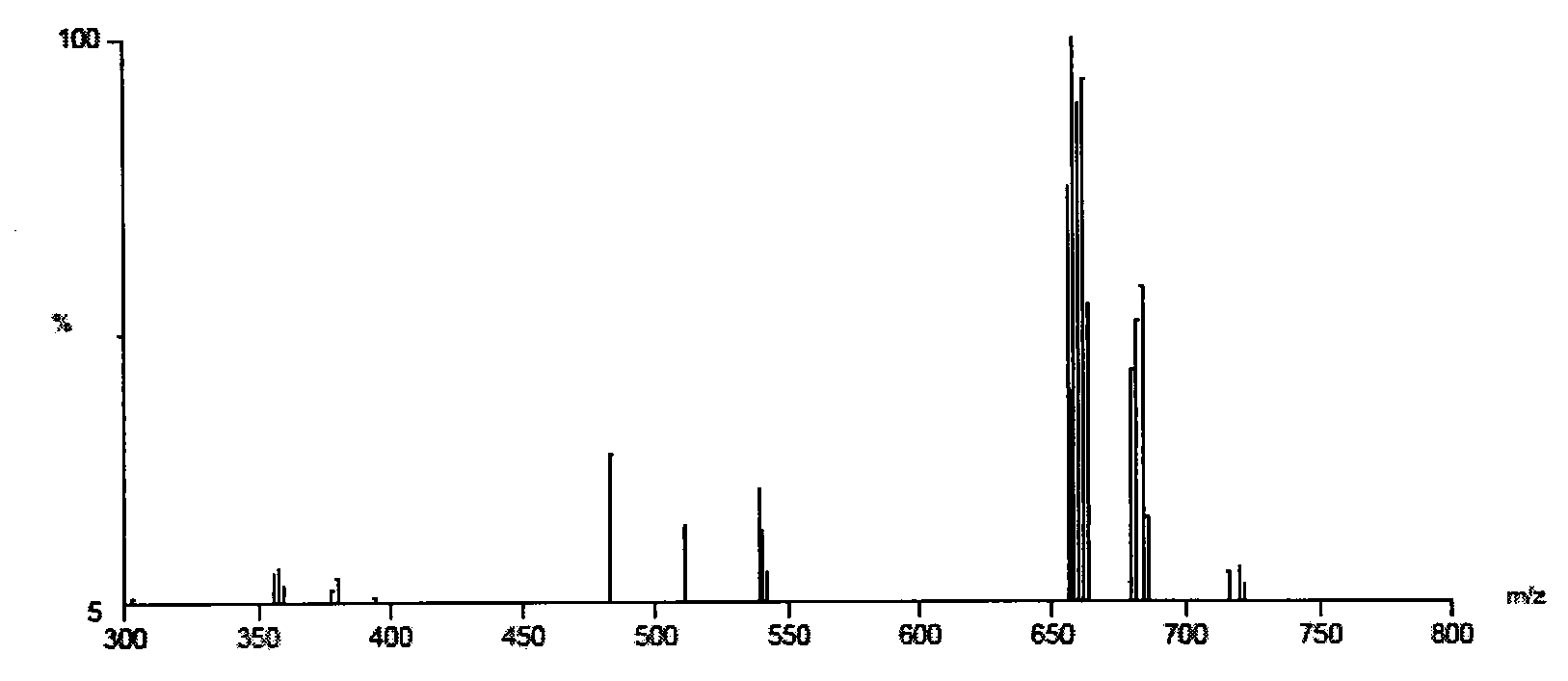
[0045] Add 6.0g of refined cardanol, 1.70g of 37%(w / w) formaldehyde solution, 0.91g of 33%(w / w) methylamine aqueous solution and 15mL of absolute ethanol to 100mL with heating, reflux, stirring device and nitrogen protection In a three-necked flask, the reaction was stirred at 70°C for 24h; the crude product from which ethanol was removed by rotary evaporation was neutralized with 5g of 30% NaOH solution, dried in a vacuum oven, washed with a proper amount of petroleum ether for several times, and then acidified with hydrochloric acid to obtain 6.12g of light pink oil. The oily substance is N,N-bis(1-cardanol methyl)-N-methylamine.
Embodiment 2
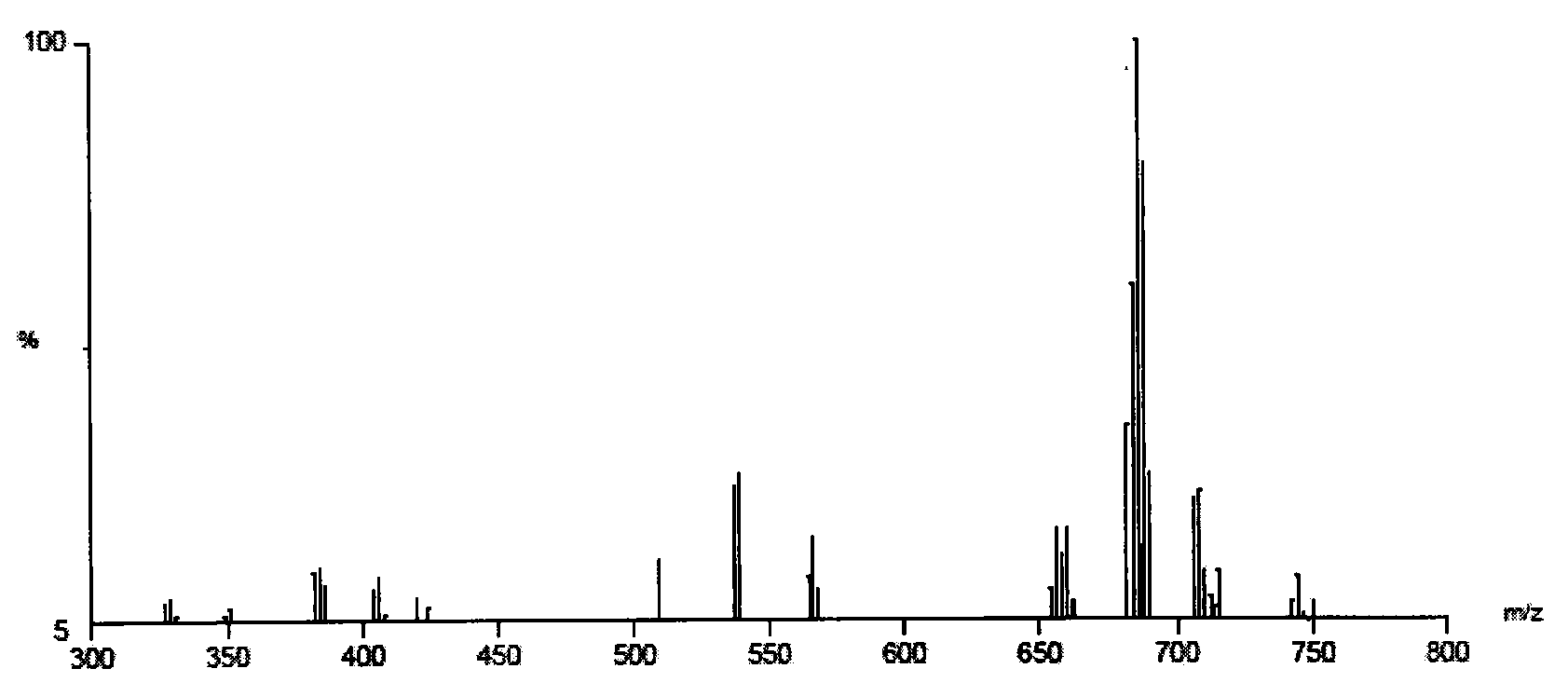
[0047] Add 6.0g refined cardanol, 0.65g trioxane, 0.73g n-butylamine and 10mL dichloromethane into a 100mL three-necked flask equipped with heating, refluxing, stirring device and nitrogen protection, heating and refluxing for reaction for 10h; The crude product from which the dichloromethane was removed was neutralized with 5g of 30% NaOH solution, dried in a vacuum oven, washed several times with an appropriate amount of petroleum ether, and then acidified with hydrochloric acid to obtain 6.70g of light pink oil, the oily substance is N,N-bis (1-Cardanol methyl)-N-butylamine.
Embodiment 3
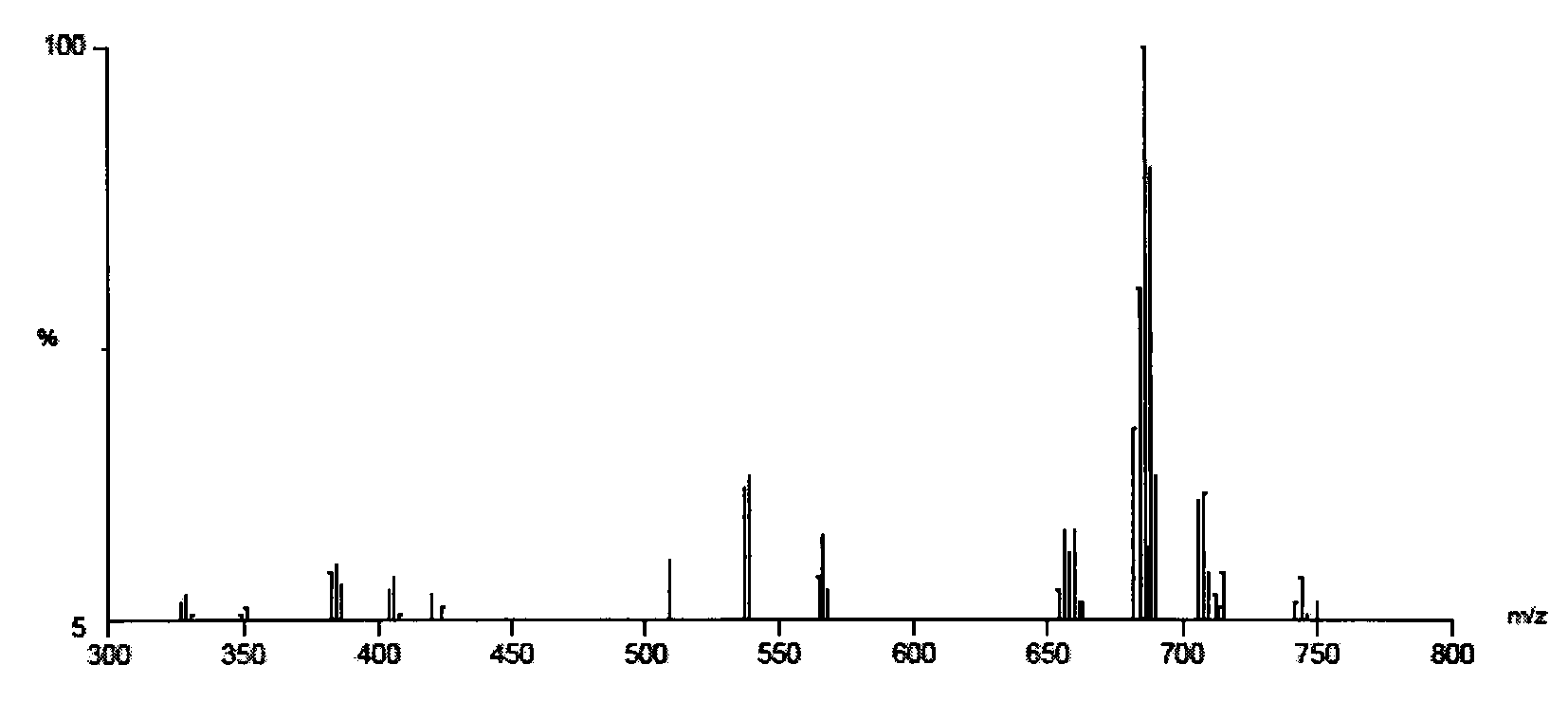
[0049] Add 6.0g of refined cardanol, 0.53g of paraformaldehyde, 1.7g of 33% (w / w) n-propylamine aqueous solution and 15mL of anhydrous methanol into a 100mL three-necked flask with heating, reflux, stirring device and nitrogen protection, heating Reflux reaction for 40h; the crude product from which methanol was removed by rotary evaporation was neutralized with 5g 30% NaOH solution, dried in a vacuum oven, washed with proper amount of petroleum ether several times, and then acidified with hydrochloric acid to obtain 5.70g pale pink oil, the oily substance is N,N-bis(1-cardanol methyl)-N-propylamine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cloud point | aaaaa | aaaaa |
| Cloud point | aaaaa | aaaaa |
| Cloud point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com