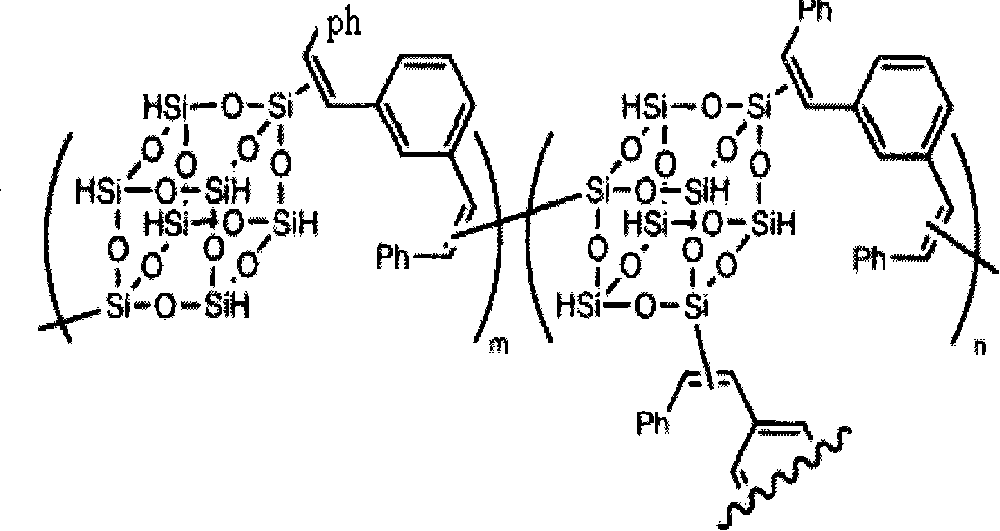
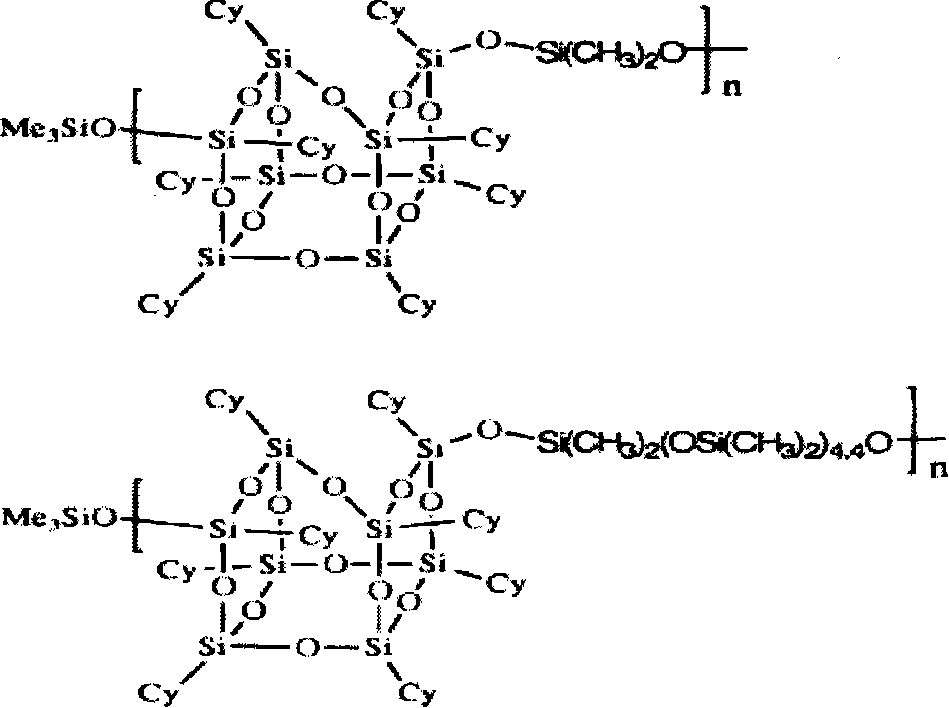
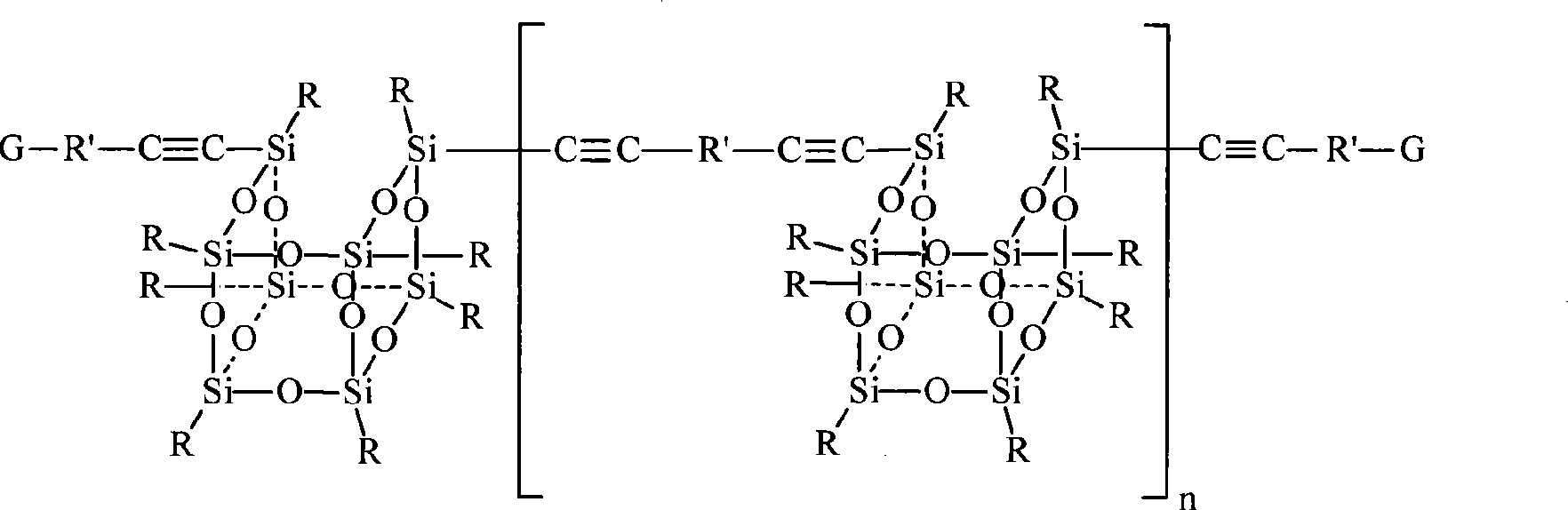Clathrate sesquialter siloxane aryne resin containing octamethyl pyrophosphoramide and method for preparing the same
A technology of octamethylcage and siloxane aryne, which is applied in the direction of silicon organic compounds, can solve the problems of difficult formation of uniform structure materials, high raw material prices, long synthesis time, etc., and achieve high thermal decomposition residual rate and mechanical properties Excellent, easy-to-apply effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of compound (II) octamethyl clathrate silsesquioxane
[0032] Add 26.81 g (0.15 mol) of methyltriethoxysilane (MTES) and 78.18 g of absolute ethanol into a 250 ml four-neck flask equipped with stirring, a constant pressure funnel, a thermometer and a spherical condenser, and add them through the constant pressure funnel. 18.00 g (1.00 mol) of ionized water and 1.10 g (0.012 mol) of tetramethylammonium hydroxide were reacted at a constant temperature of 60°C for 40 hours to obtain a yellowish solid. Suction filtration, washing with water, and vacuum drying yielded 8.33 g of a white solid with a yield of 82.6%. solid 29 Si-NMR (99MHz, 25°C) δ: -65.97 (Si-CH 3 , S); FT-IR (KBr, 25°C) v=2971.4cm -1 (CH 3 ), v=1269.63cm -1 (Si-C), γ=1411.6cm -1 (CH 3 ), v=1113.1cm - 1 (Si-O-Si).
Embodiment 2
[0033] Example 2 Synthesis of compound (III) difluorooctamethyl clathrate silsesquioxane
[0034] Add 2.59g (4.83mmol) of compound (II) octamethyl clathrate silsesquioxane and 250ml CHCl in a 500ml four-neck flask equipped with stirring, constant pressure funnel, thermometer and spherical condenser 3 , under nitrogen protection, start stirring, 4.11g (28.98mmol) of boron trifluoride diethyl ether was added dropwise to the system from a constant pressure funnel, reflux at 70°C for 30h, and 10ml of deionized water was used to terminate the reaction. Liquid separation obtains organic phase, removes solvent, obtains yellowish solid, crosses column (SiO 2 , CH 2 Cl 2 ) purification, and then recrystallized in n-hexane solution to obtain 0.50 g of white product with a yield of 18.6%. 1 H-NMR (500MHz, CDCl 3 , 25°C) δ: 0.26 (J=4.18Hz, 6H, D), 0.19 (12H, S), 0.17 (6H, S); 29 Si-NMR (99MHz, CDCl 3 , 25°C) δ: -59.79 (J=252Hz, Si-F, D), -64.40 (Si-CH 3 , S), -64.98 (Si-CH 3 , ...
Embodiment 3
[0035] Example 3 Preparation of aryne resin containing octamethyl clathrate silsesquioxane (OMPOSS-DEB-1)
[0036] Add treated 1.94g (0.0807mol) magnesium powder and 20ml THF into a 250ml four-neck flask equipped with a stirring, constant pressure funnel and a spherical condenser. Under nitrogen protection, slowly drop 5.86g (0.0538mol) through the constant pressure funnel. ) mixed solution of bromoethane and 20ml THF, 1.5h was added dropwise, and refluxed for 1.5h to obtain gray-black ethyl Grignard reagent. A mixed solution of 4.51g (0.0358mol) of diethynylbenzene and 20ml of THF was added dropwise under cooling in an ice-water bath, the reaction mixture gradually changed from grayish black to white, and the dropwise addition was completed within 1.0h, and then refluxed for 2.0h. Under cooling in a water bath, a mixed solution of 10.00 g (0.0179 mol) of compound (III) difluorooctamethyl clathrate silsesquioxane and 100 ml of THF was slowly added through a constant pressur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com