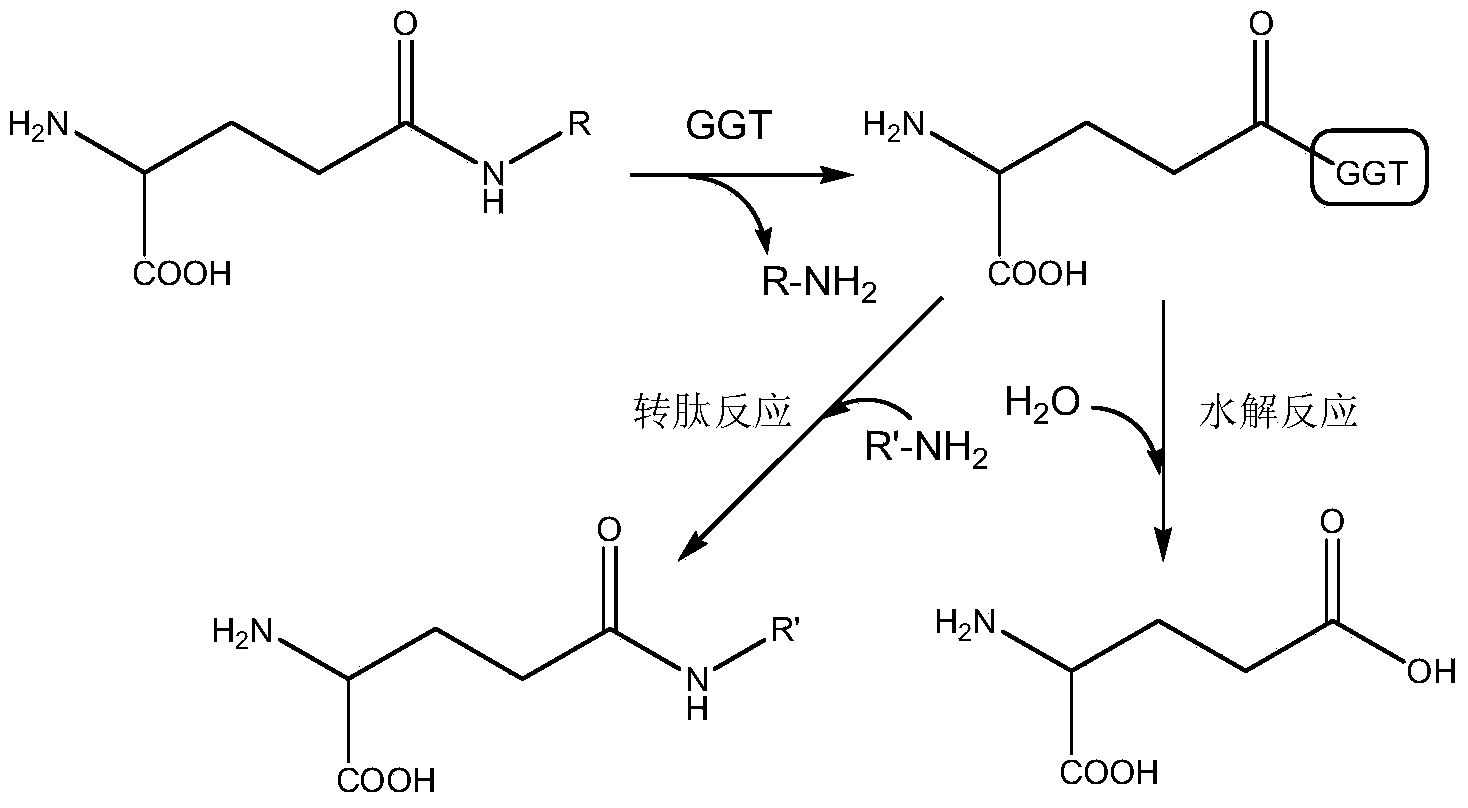
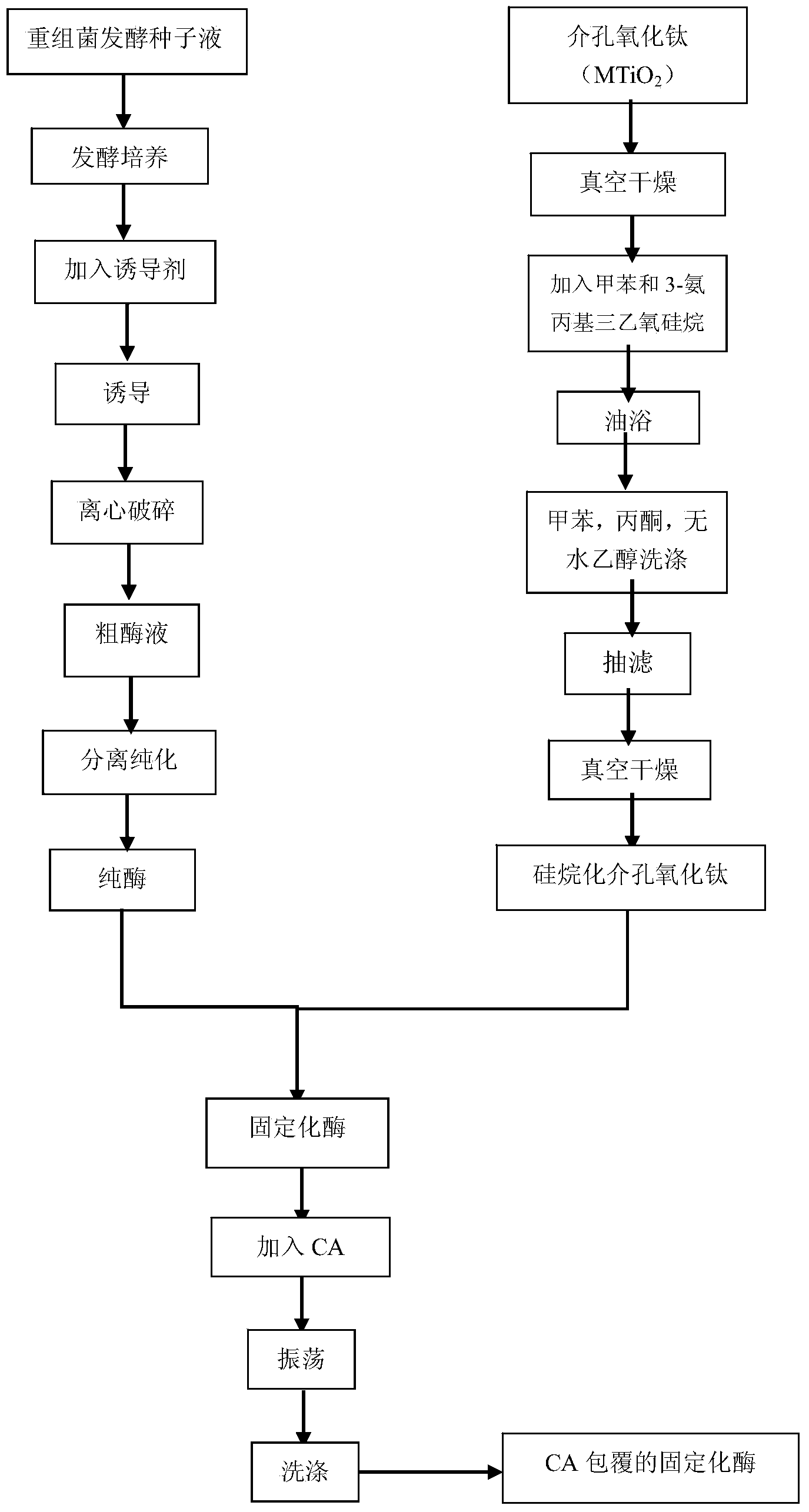
Method for modifying gamma-glutamyl transpeptidase immobilized enzyme by using carrier ampholyte
A technology of glutamyl transpeptidase and ampholyte, which is applied in the direction of immobilization on or in the inorganic carrier, can solve the problems of low success rate, high cost, and long cycle, and achieve improved pH stability and strong buffering The effect of large capacity and specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]After fermenting and culturing the recombinant Escherichia coli for 12 hours, add the inducer lactose 2g / L, induce for 20 hours at 37°C, centrifuge, wash and ultrasonically break at a power of 400w, and centrifuge to obtain the supernatant to obtain a crude enzyme solution at 0% to 50%, 50% to 80%, 80% to 100% saturation ammonium sulfate gradient precipitation. Take 80%~100% active protein precipitate and redissolve it with Tri-HCl buffer (pH8.0, 50mmol / L). After dialysis, use DEAE Sepharose Fast Flow ion exchange chromatography and Source15Q ion exchange chromatography to obtain pure enzyme solution . Mesoporous titanium oxide (the specific surface area of mesoporous titanium oxide is 96.539m 2 / g) placed in an oven at 120°C for 10 hours in vacuum to constant weight. Take 1g and put it into a 250mL round bottom flask, then add 100mL toluene and 1.47mL APTES, and react in an oil bath at 100°C under reflux for 6h. After the reaction is over, wash the powder with 250 ...
Embodiment 2
[0034] After fermenting and culturing the recombinant Escherichia coli for 12 hours, add the inducer lactose 2g / L, induce for 16 hours at 23°C, centrifuge, wash, and ultrasonically break at 400w power, centrifuge to get the supernatant to obtain a crude enzyme solution at 0% to 50%, 50% to 80%, 80% to 100% saturation ammonium sulfate gradient precipitation. Take 80%~100% active protein precipitate and redissolve it with Tri-HCl buffer (pH8.0, 50mmol / L). After dialysis, use DEAE Sepharose Fast Flow ion exchange chromatography and Source15Q ion exchange chromatography to obtain pure enzyme solution . Mesoporous titanium oxide (the specific surface area of mesoporous titanium oxide is 96.539m 2 / g) placed in an oven at 100°C for 10 hours in vacuum to constant weight. Take 1g and put it into a 250mL round bottom flask, then add 80mL toluene and 1mL APTES, and react in an oil bath at 60°C under reflux for 6h. After the reaction is over, wash the powder with 250 mL of toluene, ...
Embodiment 3
[0039] After fermenting and culturing the recombinant Escherichia coli for 10 hours, add the inducer lactose 3g / L, induce for 15 hours at 25°C, centrifuge, wash and ultrasonically break at 400w power, and centrifuge to obtain the supernatant to obtain a crude enzyme solution at 0% to 50%, 50% to 80%, 80% to 100% saturation ammonium sulfate gradient precipitation. Take 80%~100% active protein precipitate and redissolve it with Tri-HCl buffer (pH8.0, 50mmol / L). After dialysis, use DEAE Sepharose Fast Flow ion exchange chromatography and Source15Q ion exchange chromatography to obtain pure enzyme solution . Mesoporous titanium oxide (the specific surface area of mesoporous titanium oxide is 96.539m 2 / g) placed in a 100°C oven for 8 hours to a constant weight. Take 1g and add it to a 250mL round bottom flask, then add 80mL toluene and 1mL APTES, and react in an oil bath at 50°C under reflux for 5h. After the reaction is over, wash the powder with 250 mL of toluene, acetone, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com