Method for detecting activity of beta-1,4-xylosyltransferase in xylan synthesis by utilizing high performance liquid chromatography (HPLC)
A technology for xylose transferase and xylan, which is applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of difficult operation and cumbersome methods, and achieve the effects of simple operation, high sensitivity and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1 Xylo-oligosaccharides containing fluorescent groups (Xyl n -AA) mark
[0056] ① Preparation of derivatization reaction reagents: 30mg / mL fluorescent group 2-aminobenzoic acid (Anthranilic Acid, AA) and 20mg / mL sodium cyanoborohydride (NaBH 3 CN) was dissolved in MABS to obtain the derivative reaction
[0057] Reagents; Among them, MABS (Methanol, Acetate, Borate mixed solution)
[0058] For: 24mg / mL sodium acetate and 20mg / mL boric acid are dissolved in methanol solution, and MABS should be prepared and used immediately;
[0059] ② Labeling reaction: xylooligosaccharide (Xyl n , n is 1 to 6) (purchased from the Carbohydrate Research Center of the University of Georgia, USA, http: / / www.ccrc.uga.edu / services / index.php) and the derivatization reaction reagent obtained in step ① according to the mass volume ratio of 1mg : Mix 1mL, react at 80°C for 80min, add diethyl ether (diethyl ether, analytically pure) to mix the excess derivatizing reagent and extract...
Embodiment 2
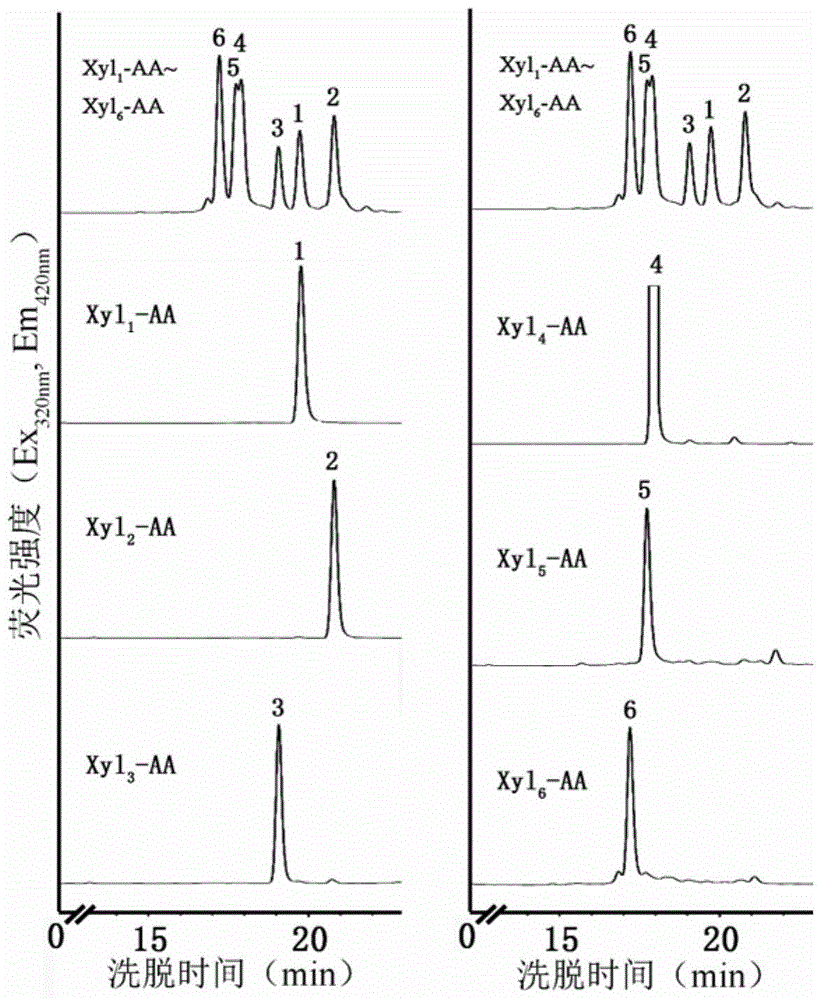
[0061] Embodiment 2 establishes HPLC to detect Xyl n -AA method
[0062] (1) Preparation of Xyl 1 -AA~Xyl 6 - Individual standard samples and mixed standard samples of AA, each with a final concentration of 0.1 mM.
[0063] (2) Determine the peak elution time of each standard sample and mixed standard samples by HPLC. The chromatographic conditions are: chromatographic column ZORBAX Eclipse XDB-C18 column (2.1mm×250mm, 1.8μm) (Agilent, purchased from Agilent Technologies Co., Ltd.), mobile phase: A phase is 50mM sodium acetate aqueous solution (pH4.3), B phase Chromatographically pure acetonitrile (Honeywell, 99.99% pure, purchased from Shanghai Dingguo Biotechnology Co., Ltd.), flow rate: 0.5mL / min, column temperature: 20°C, detector: Agilent1100HPLC systems (purchased from Agilent Technologies Ltd.), Ex 320nm ,Em 420nm , injection volume 5μL, recording time: 42min. Gradient elution program: 0~5min (8% phase B), 5~25min (20% phase B), 25~30min (40% phase B), 30~35min (...
Embodiment 3
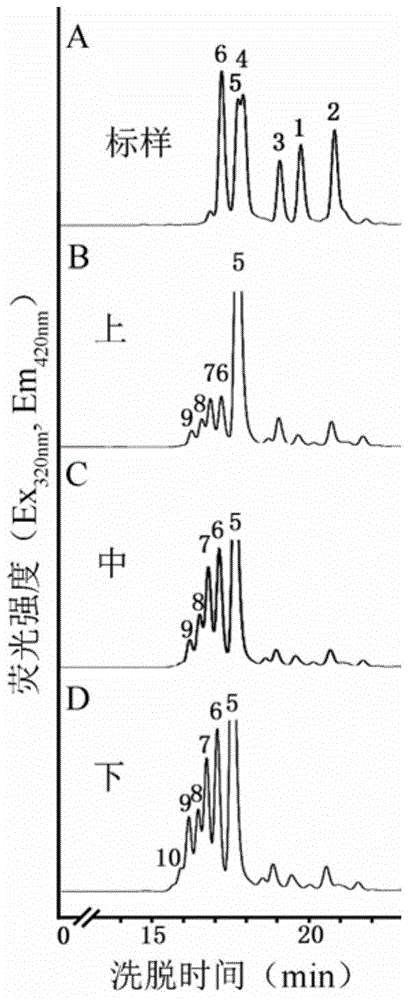
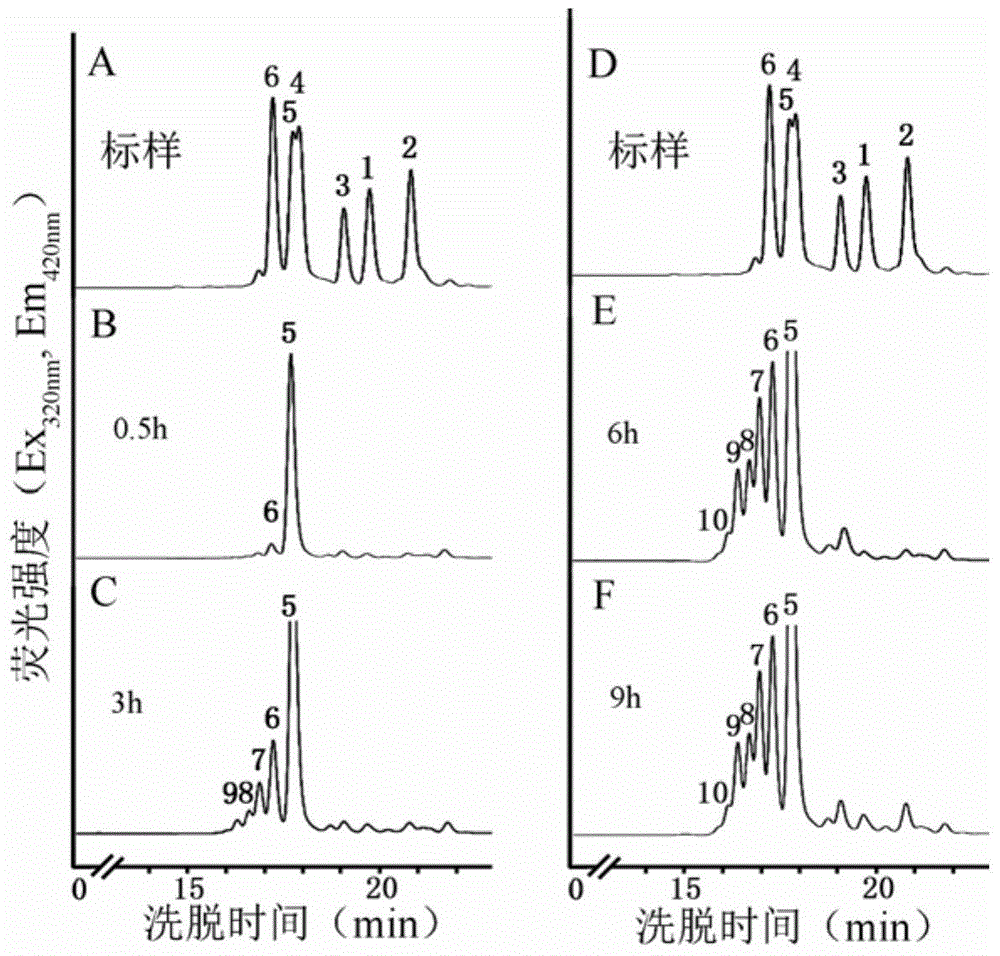
[0066] Example 3 Analysis of XylT activity of japonica
[0067] 1. Extraction of yellow beam wood microcapsules
[0068] Take the peeled stem segments of the upper, middle and lower parts of the annual Neolamarckia cadamba (Rubiaceae) (collected in Tianhe Park, Guangzhou), and add them to the extract solution (100mM Hepes-KOHbuffer pH6.8, 1mM DTT, 1mM EDTA , 0.1 mM MnCl 2 , 0.4M sucrose; Among them, the Hepes-KOH buffer of 100mM pH6.8 is the solution of 23.831g 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) dissolved in deionized water, and the volume is adjusted to 1L, and the 100mM Hepes solution is adjusted with KOH pH to 6.8. ) was homogenized in a high-speed tissue grinder (model DS-1, purchased from Shanghai Specimen Model Factory) at 4°C (the weight-to-volume ratio of the plant to the extract was 2g: 1mL), and filtered through a filter cloth (cat.475855, Miracloth, Calbiochem Company) for filtration, the obtained filtrate was centrifuged at 10,000g, 4°C for 10mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com