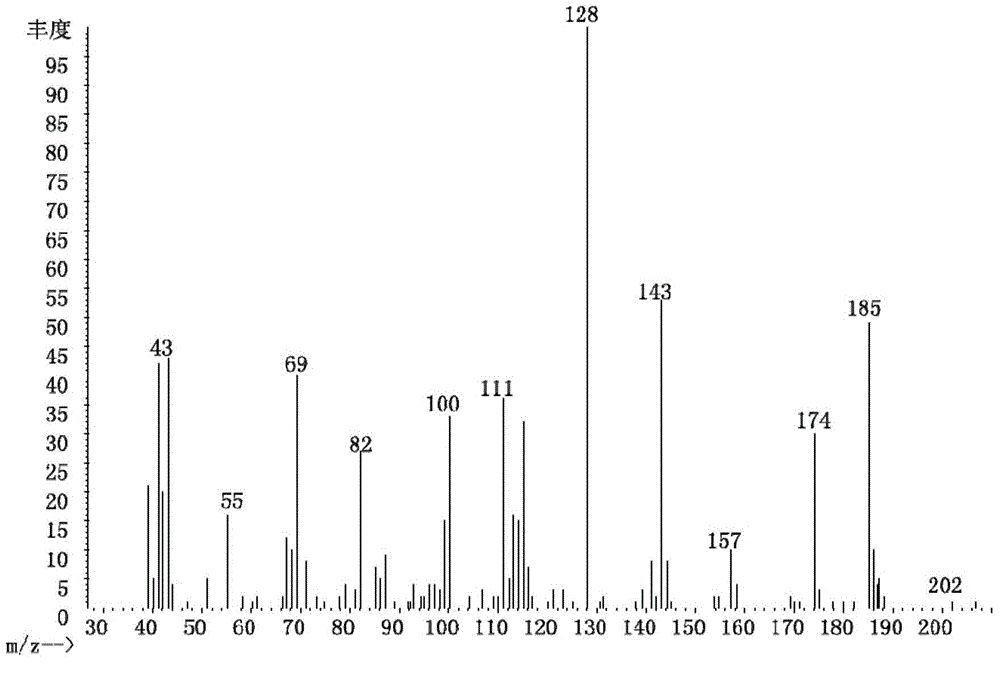
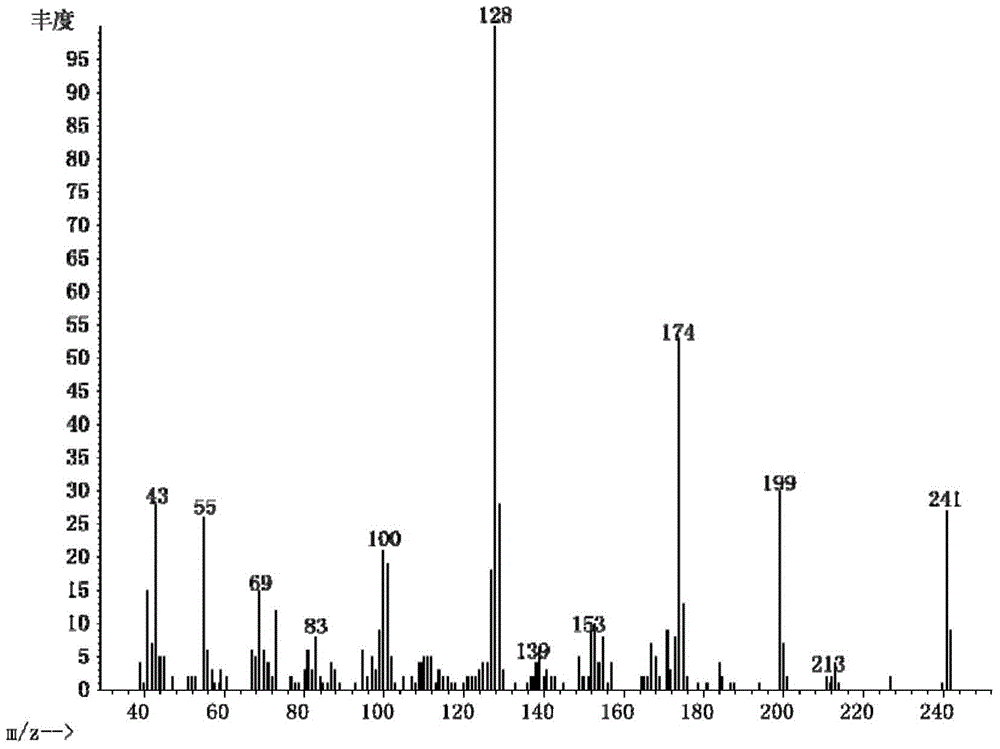
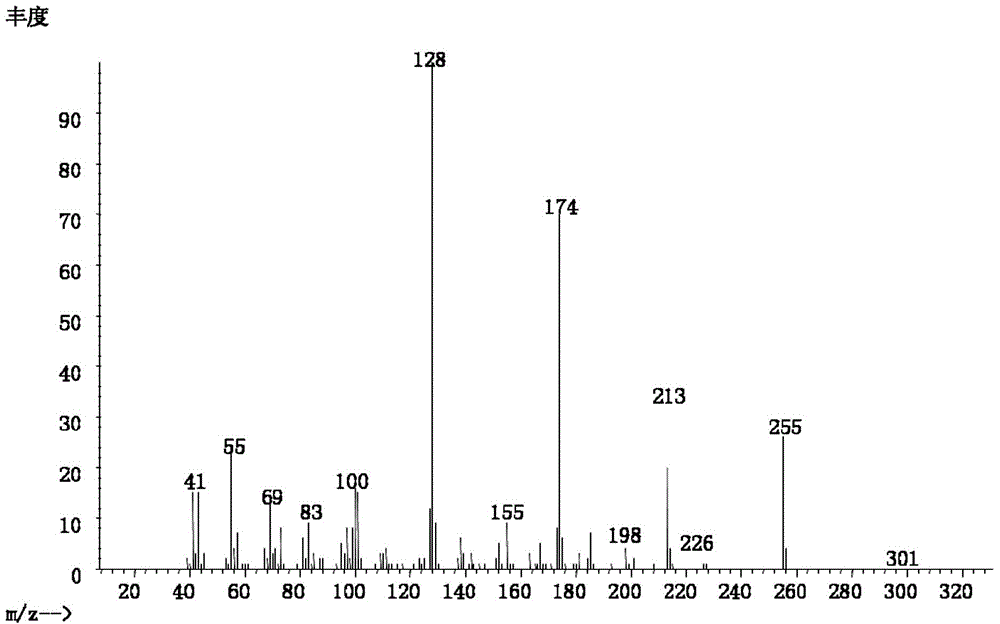
Method for preparing 2-(1-methylalkyl) succinic acid
A technology of methyl alkyl and succinic acid, applied in the preparation of carboxylate, the preparation of organic compounds, the preparation of carboxylate/lactone, etc., can solve the problem of harsh reaction conditions, expensive catalysts, and unavailability of reaction raw materials, etc. problem, to achieve the effect of less by-products and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of 2-(1-methylethyl)succinic acid
[0042](1) In a 100mL flask, mix 0.4mol 1-isopropanol, 0.4mol HBr and 0.4mol H 2 SO 4 After mixing at 0°C, react at 120°C for 1 h, dilute with water and extract with ether three times after cooling, remove the solvent by evaporation to obtain 1-bromoisopropane.
[0043] (2) In a 100mL flask, cut 0.4mol Na into small pieces and add it to 30mL absolute ethanol, stir at 0°C until no H 2 Generated to produce sodium ethoxide / ethanol solution. Add 0.4mol diethyl malonate to the solution, move to room temperature and stir for 0.5h. Then, 0.4 mol ethyl bromoacetate was added to the solution at 0°C. After the addition was completed, it was stirred at 0°C for 4h and at room temperature overnight. After the reaction, the ethanol was distilled off, a saturated NaCl aqueous solution was added, the pH of the solution was adjusted to <1 with dilute HCl, and the product was extracted three times with ethyl acetate. ...
Embodiment 2
[0047] Embodiment 2: the preparation of 2-(1-methylheptyl) succinic acid
[0048] (1) In a 100mL flask, mix 0.4mol1-n-pentanol, 0.42mol HBr and 0.48mol H 2 SO 4 After mixing at 0°C, react at 120°C for 2h, dilute with water and extract with petroleum ether three times after cooling, remove the solvent by evaporation to obtain 1-bromo-n-pentane.
[0049] (2) Cut 0.52mol Na into small pieces and add it to 30mL of absolute ethanol, at 0°C until there is no H 2 Generated to produce sodium ethoxide / ethanol solution. Add 0.48mL of ethyl acetoacetate to the solution, react at 0°C for 0.8h. Then add 0.4mol 1-bromo-n-pentane to the solution, and react at 75°C until the white solid no longer increases. After the reaction, distill off ethanol, add saturated NaCl aqueous solution, adjust pH<1 with dilute HCl solution, and extract 3 times with ethyl acetate. After combining the organic phases, the ethyl acetate was removed by evaporation to give ethyl 2-pentylacetoacetate.
[0050] (3...
Embodiment 3
[0057] Embodiment 3: the preparation of 2-(1-methyloctyl) succinic acid
[0058] (1) In a 100mL flask, mix 0.4mol 1-n-hexanol, 0.48mol HBr and 0.56mol H 2 SO 4 After mixing at 0°C, react at 120°C for 3h, dilute with water and extract with petroleum ether three times after cooling, remove the solvent by evaporation to obtain 1-bromo-n-hexane.
[0059] (2) Cut 0.4mol Na into small pieces and add it to 30mL of absolute ethanol, at 0°C until there is no H 2 Generated to produce sodium ethoxide / ethanol solution. Add 0.4 mL of ethyl acetoacetate to the solution, and stir at 0° C. for 0.5 h. Then add 0.4 mol of 1-bromo-n-hexane to the solution, and react at 70° C. until the white solid no longer increases. After the reaction, distill off ethanol, add saturated NaCl aqueous solution, adjust pH<1 with dilute HCl solution, and extract 3 times with ethyl acetate. After combining the organic phases, the ethyl acetate was removed by evaporation to give ethyl 2-hexylacetoacetate.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com