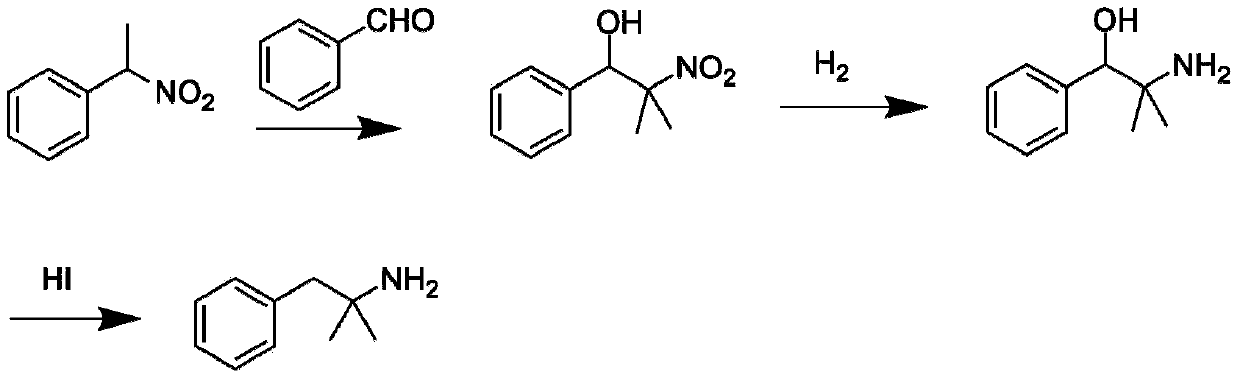
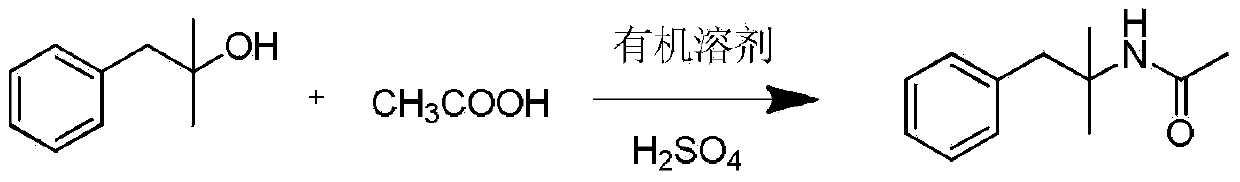
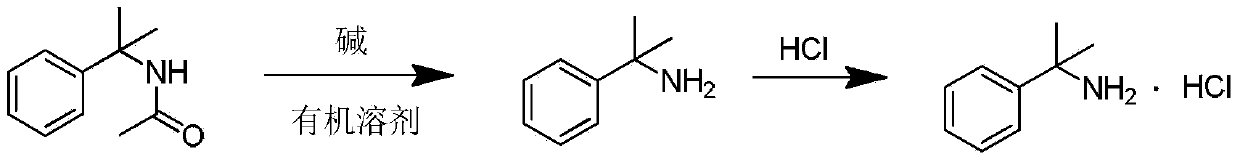
Synthesis method of phentermine hydrochloride
A synthesis method and phentermine technology, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of reducing production efficiency, high equipment requirements, long reaction time, etc., and meet the requirements of reducing equipment , the reaction conditions are safe, and the effect of reducing the probability of occurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a. Add 2.31g CH to a 250mL three-necked bottle 3 COOH (38.5mmol), 4.80gCH 3 CN (117.01mmol) was stirred evenly, and 5.67g of dimethylbenzyl alcohol (37.75mmol) was added dropwise to the three-necked flask at a rate of 1 drop / s under ice-water bath conditions. After the dropwise addition, 1 mL of concentrated sulfuric acid with a molar concentration of 18.4 mol / L was slowly added dropwise, the ice-water bath was removed, and the reaction was stirred for 2 h, and monitored by TLC until the raw material point completely disappeared.
[0035] b. Add 20 mL of water to the completed reaction flask until it becomes turbid, then add 1 mol / L NaOH solution to adjust the pH to 6.2. Solids are precipitated, filtered under reduced pressure, and then washed with water to obtain N-(1,1- Dimethyl-phenethyl)acetamide 6.88g, yield 95.30%.
[0036] c. Dissolve 6.88g of N-(1,1-dimethyl-phenylethyl)acetamide (38.82mmol) in 20mL of ethylene glycol until completely dissolved, add 2.22g of p...
Embodiment 2
[0040] a. Add 49.97g CH to a 250mL three-necked bottle 3 COOH (832.13mmol), 68.32g of n-butyronitrile (1.66mol) were dissolved, and 25.0g of dimethyl benzyl alcohol (166.43mmol) was added dropwise to the three-necked in the bottle. After the dropwise addition, 4 mL of concentrated sulfuric acid with a molar concentration of 18.4 mol / L was slowly added dropwise, the ice bath was removed, and the reaction was stirred for 8 hours, and monitored by TLC until the raw material point completely disappeared.
[0041] b. Pour the contents of the reaction bottle into a 500mL beaker, add 100mL of water, the solution is turbid, add 3mol / L KOH solution to adjust the pH to 7.1, solids are precipitated, vacuum filtration, and then wash the solids with water to obtain N -(1,1-Dimethyl-phenethyl)acetamide 30.7g, yield 96.44%.
[0042] c. Dissolve 30.7g of N-(1,1-dimethyl-phenylethyl)acetamide (173.21mmol) in 200mL of n-butanol, add 20.78g of potassium hydroxide (519.63mmol), and heat to refl...
Embodiment 3
[0046] a. Add 167.9g CH to a 1L three-necked bottle 3 COOH (4.61mol), 382.42gC 2 h 5CN (6.94mol) was dissolved, and 70.0g of dimethylbenzyl alcohol (465.99mmol) was dropped into the three-necked flask at a rate of 2-3 drops per second under ice-bath conditions. After the dropwise addition was completed, 10 mL of concentrated sulfuric acid with a molar concentration of 18.4 mol / L was slowly added dropwise, the ice bath was removed, and the reaction was stirred for 15 hours. TLC monitored until the raw material point completely disappeared.
[0047] b. Pour the contents of the reaction bottle into a 2L beaker, add 500mL of water, the solution becomes cloudy, and then add 6mol / L of Ba(OH) 2 The pH of the solution was adjusted to 7.9, and a solid precipitated out. The solid was filtered under reduced pressure, and the solid was washed with water to obtain 84.6 g of N-(1,1-dimethyl-phenethyl)acetamide, with a yield of 94.92%.
[0048] c. Dissolve 84.6g of N-(1,1-dimethyl-pheneth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com