Electrochemical sensor for detecting lead as well as preparation method and application thereof
An electrochemical and sensor technology, applied in the field of electrochemical sensors for detecting lead and its preparation, can solve the problems of unstable fixation, affecting DNA activity, and easy use of environmentally harmful substances, so as to improve sensitivity, improve detection performance, and improve The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
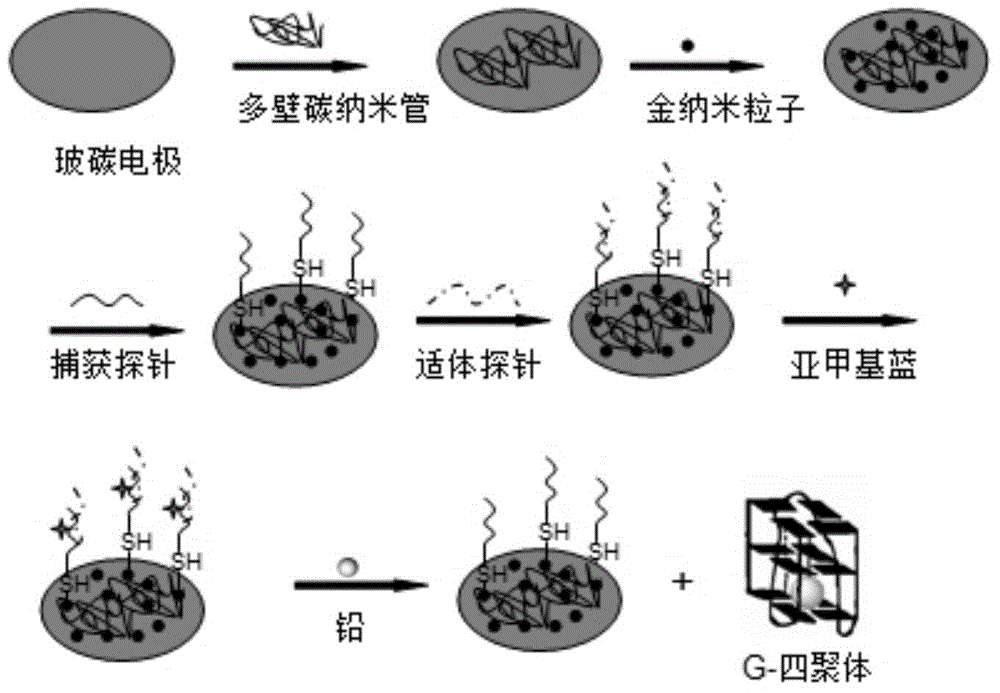
[0040] see figure 1 , an electrochemical sensor for the detection of lead ions, comprising a glassy carbon electrode used as a working electrode in a three-electrode system, an aptamer probe, and methylene blue. The surface of the detection end of the glassy carbon electrode is modified with multi-walled carbon nanotubes, nano-gold particles are deposited on the multi-walled carbon nanotubes, and thiol-modified capture probes are connected to the nano-gold particles. When the electrochemical sensor is used to detect lead ions, the aptamer probe and the sulfhydryl-modified capture probe form a double-stranded structure through complementary pairing, and methylene blue (0.1 mM concentration) is embedded in the sulfhydryl-modified capture probe and aptamer probe. in the double-stranded structure formed.
[0041] The concentration of methylene blue can also be 0.1-0.5 mM.
[0042] In the present invention, the capture probe and the aptamer probe can be any amino acid sequence th...
Embodiment 2
[0048] The preparation method of the electrochemical sensor of embodiment 1.
[0049] Polish the surface of the glassy carbon electrode, then wash the surface of the glassy carbon electrode with water, then use nitric acid, acetone, and water to ultrasonically clean it, and finally use a concentration of 10mM Tris-HCl buffer solution (Tris-HCl buffer solution contains 1.0M KCl ) rinsed, dried naturally, and then used for the preparation of electrochemical sensors, the specific preparation method is:
[0050] S1. Carboxylate the multi-walled carbon nanotubes to obtain carboxylated multi-walled carbon nanotubes; the specific steps are:
[0051] The multi-walled carbon nanotubes are immersed in a mixed solution with a volume ratio of 1:3 of hydrogen peroxide and concentrated sulfuric acid (the mass fraction of concentrated sulfuric acid is 98%) (the volume ratio of hydrogen peroxide and concentrated sulfuric acid can also be 1:2 ~4), ultrasonication at a temperature of 50°C for ...
Embodiment 3
[0060] The application of the electrochemical sensor of embodiment 1 in detecting lead ion, concrete detection method is:
[0061] Add the aptamer probe dropwise to the reaction end surface of the glassy carbon electrode of the electrochemical sensor, react at 37°C for 60 minutes, and then drop the methylene blue with a concentration of 0.1mM (the concentration of methylene blue can be 0.1-0.5mM) on the glassy carbon electrode The surface of the reaction end was reacted at 37°C for 20 minutes; then the concentration of lead ions was respectively adjusted to 5.0×10 -11 M~1.0×10 -14 The test solution of M was added dropwise on the surface of the glassy carbon electrode, and after 30 minutes of reaction, it was connected to the electrolytic cell of the three-electrode system, and the current value was detected with Tris-HCl with a pH of 7.4 as the electrolyte solution.
[0062] Figure 4 is the concentration of lead ions are 0M(a), 1.0×10 -14 M(b), 5.0×10 -14 M(c), 1.0×10 -1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com