A polyamide antibacterial agent, a preparing method and applications thereof
An antibacterial agent and polyamide technology, which is applied in the field of preparation, antibacterial finishing of fabrics, and polyamide antibacterial agents, can solve the problems of antibacterial activity and durability, and achieve superior comprehensive finishing performance, convenient dispersion, and easy access to raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1, N -(3-Triethoxysilylpropyl)- N′ -( N -Heptylurea- N″ -Ethyl)succinamide Synthesis
[0042] (1) N- (3 - Synthesis of triethoxysilylpropyl)-carboxypropyl formamide
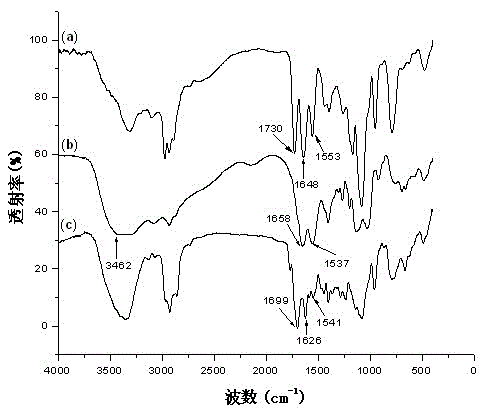
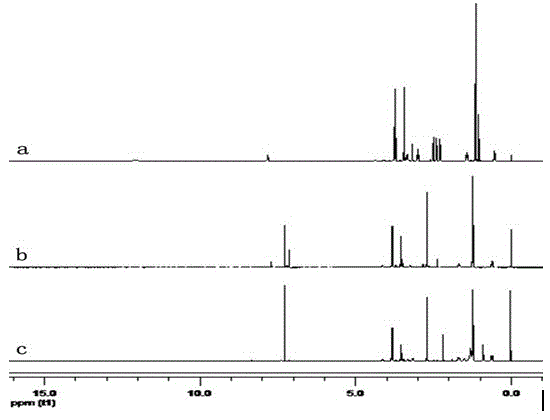
[0043] 22.1 g of 3-aminopropyltriethoxysilane (APTES), 100 g of tetrahydrofuran (THF) and 10 g of succinic anhydride were added into a 1000 mL three-necked flask, under nitrogen protection, and reacted at room temperature for 18 h. Wash with methanol, filter to remove a small amount of solid impurities, and remove the solvent to obtain a colorless, transparent and viscous compound N- (3 - Triethoxysilylpropyl)-carboxypropyl formamide. FTIR (cm -1 ): 3367 (NH), 3293 (OH), 2974 (CH 3 ), 2932 (CH 2 ), 2886 (CH 2 ), 1730 (CO, acid), 1648 (CO, amidⅠ), 1553 (NH, amideⅡ), 1080 (Si-O-Et). 1 H NMR (ppm) (400 MHz, DMSO-d 6 ): δ 0.56 (t, J =4.8 Hz, 2H, Si-C H 2 -), 1.16 (t, J =4.6Hz, 9H, -C H 3 ), 1.41-1.46 (m, J =5.0 Hz, 2H, CH 2 -C H 2 -CH 2 -), 2.29 (t, J =6.6 Hz, 2H, CO-C H 2 )...
Embodiment 2
[0057] 1, N -(3-Trimethoxysilylpropyl)- N′ -( N -Heptylurea- N″ -Ethyl)succinamide Synthesis
[0058] (1) N- (3 - Synthesis of trimethoxysilylpropyl)-carboxypropyl formamide
[0059] Add 16.8 g of 3-aminopropyltrimethoxysilane (APTMS), 150 g of tetrahydrofuran (THF) and 10 g of succinic anhydride into a 500 mL three-necked flask, and react at room temperature for 18 h under nitrogen protection. Wash with methanol, filter to remove a small amount of solid impurities, and remove the solvent to obtain a colorless, transparent and viscous compound N- (3 - Trimethoxysilylpropyl)-carboxypropyl formamide. FTIR (cm -1 ): 3366 (NH), 3293 (OH), 2977 (CH 3 ), 2930~2888 (CH 2 ), 1731 (-C=O, acid), 1649 (-C=O, amidⅠ), 1553 (NH, amideⅡ), 1081 (Si-O-Et).
[0060] (2) N -(3-Trimethoxysilylpropyl)- N′ - Synthesis of aminoethyl-succinamide
[0061] Add 27.5 g to a 500 mL three-neck flask N- (3 - Trimethoxysilylpropyl)-carboxypropyl formamide and 150 g tetrahydrofuran, unde...
Embodiment 3
[0070] 1, N -(3-Triethoxysilylpropyl)- N′ -( N -Lauryl urea- N″ -Ethyl)succinamide Synthesis
[0071]In the 500 mL three-necked flask, add the obtained in embodiment 1 N -(3-Triethoxysilylpropyl)- N′ -Aminoethyl-succinamide 7.26 g and 50 g dehydration acetone, react at 45 ℃, add 0.1 g dibutyltin dilaurate (DBTDL) dropwise, slowly add 6.18 g dodecyl isocyanate in 80 g acetone solution ( 30 min dropwise), reflux for 2 h. Acetone was removed under reduced pressure to obtain light yellow oily substance N -(3-Triethoxysilylpropyl)- N′ -( N -Lauryl urea- N″ - ethyl) succinamide. FTIR (cm -1 ): 3335~3329 (NH), 2920~2856 (CH 2 ), 1698 (CO, amidⅠ), 1625 (CO, carbamido), 1542 (NH, amideⅡ), 1079 (Si-O-Et).
[0072] The molecular structure of the product is as follows:
[0073] .
[0074] According to antibacterial finishing and antibacterial property test in embodiment 1, record antibacterial cotton fabric to Gram-negative bacteria escherichia coli Escherichia coli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com