Preparation method for honeysuckle extract
A technology of honeysuckle extract and medicinal materials, which is applied in the field of preparation of honeysuckle extract, can solve the problems of inability to comprehensively understand the medicinal effect and detection of honeysuckle extract, and achieve the effect of overall transfer, low preparation cost, and controllable conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
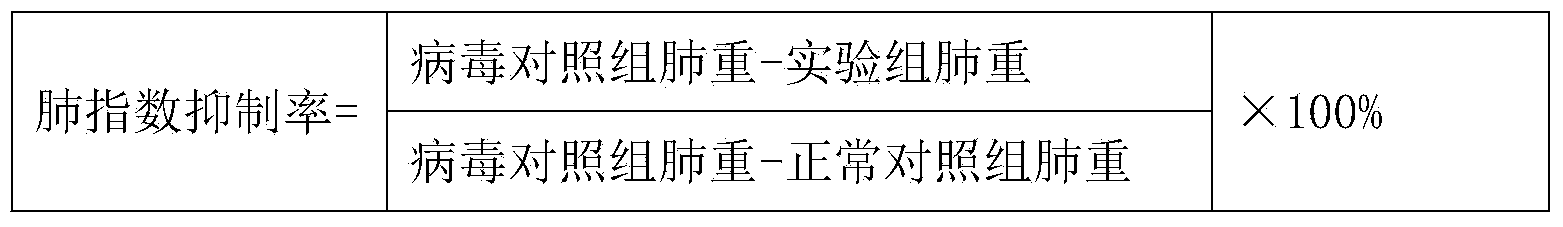
[0028] Example 1 Anti-influenza A H1N1 Influenza Virus Test of Honeysuckle Extract
[0029] 1 Test purpose
[0030] To investigate the pharmacodynamics of honeysuckle extract by using influenza A H1N1 influenza virus infection mouse model of pneumonia.
[0031] 2 Test materials
[0032] 2.1 Test drugs
[0033] Honeysuckle extract, batch number: 20111927 (the honeysuckle extract obtained in Example 13 of the present invention), brown-black powder, bitter smell, crisp texture, soluble in water and ethanol.
[0034] 2.2 Positive control drug Yinhuang Oral Liquid: produced by Lunan Houpu Pharmaceutical Co., Ltd.; national drug approval: Z10870001; production batch number: 09122001; production date: 091203; valid until 201211; properties: reddish-brown clear liquid, sweet, Slightly bitter; functions and indications: clearing heat and dispelling wind, relieving sore throat and detoxifying. It is used for dry throat, sore throat, swollen laryngeal nucleus, thirst, and fever cause...
Embodiment 2
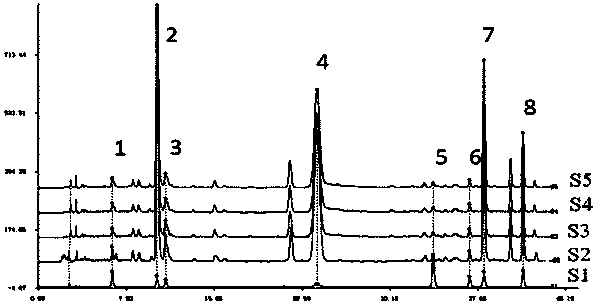
[0053] Example 2 Establishment of the Fingerprint of Honeysuckle Extract
[0054] 1 Instruments and reagents
[0055] Agilent1100 high-performance liquid chromatography (Agilent Technology Co., Ltd.), AG285 analytical balance (Mettler Toledo); pHS-25 acidity meter (Shanghai Weiye Instrument Factory); glass chromatography column (1cm × 50cm).
[0056] Honeysuckle, purchased from Jiujianpeng Honeysuckle Professional Cooperative; Luteoloside (luteoloside, purity ≥ 98%, batch number 111720-201307, China Institute for Food and Drug Control); Chlorogenic acid (3-O-CQA, purity ≥ 98%, Batch number 110753-200413, China Institute for Food and Drug Control); neochlorogenic acid, cryptochlorogenic acid (5-O-CQA, 4-O-CQA, purity ≥ 98%, batch numbers are MUST-10091501, MUST-10091811, Chengdu Master Biological Technology Co., Ltd.); 4,5-O-dicaffeoylquinic acid, 3,5-O-dicaffeoylquinic acid, 3,4-O-dicaffeoylquinic acid (4 ,5-DCQA, 3,5-DCQA, 3,4-DCQA, purity ≥ 98%, batch numbers are 12022804,...
Embodiment 3
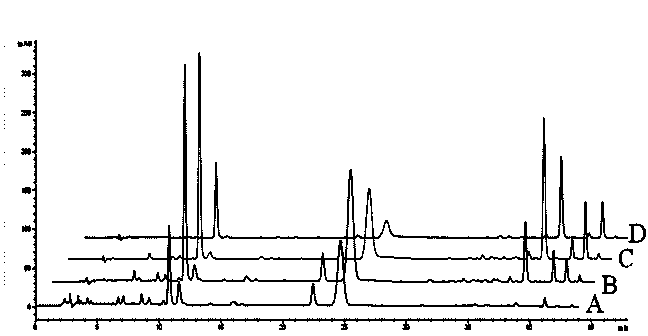
[0067] Embodiment 3 Macroporous resin purification process research
[0068] 2.4 Purification of macroporous resin
[0069] 2.4.1 Resin pretreatment Soak the resin in absolute ethanol for 24 hours, put it into a glass chromatography column, wash it with absolute ethanol at a flow rate of 2BV / h until the effluent is not turbid after adding deionized water, and then wash it with deionized water Rinse until there is no alcohol smell, then rinse with 2BV5% dilute hydrochloric acid solution at a flow rate of 2BV / h, then rinse the resin with deionized water until the pH of the effluent is neutral, and finally use 2BV2% sodium hydroxide solution at a flow rate of 2BV / h After rinsing, rinse the resin with deionized water until the pH value of the effluent is neutral, filter with suction, and remove the water for later use.
[0070] 2.4.2 Resin Screening Accurately weigh 5.0g of the pretreated resin into a stoppered Erlenmeyer flask, accurately add 40mL of honeysuckle extract, place i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com