High performance liquid chromatography detecting method for abiraterone acetate and tablet of abiraterone acetate
A technology of abiraterone acetate and high performance liquid chromatography, which is applied to measurement devices, instruments, scientific instruments and other directions, can solve the problems of poor crystal fluidity, unfavorable crystal form stable storage, crystal form damage threat, and the like, and achieves reproducibility. The effect of good performance, strong specificity and high analytical sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0175] Abiraterone Acetate Tablets
[0176] Component
%(w / w)
34.97
lactose monohydrate
27.82
21.21
10.00
povidone
3.50
1.00
1.50
[0177] 1. Preparation process:
[0178] 1.1 Preparation before ingredients
[0179] 1.1.1 Screening
[0180] Get the crushed abiraterone acetate and pass through a 60-mesh sieve for subsequent use.
[0181] 1.2 Ingredients
[0182] Picking and nuclear materials are carried out according to the batch feeding quantity.
[0183] 1.3 Hybrid
[0184] Weigh the prescribed amount of abiraterone acetate, 0.5% sodium lauryl sulfate, lactose monohydrate, microcrystalline cellulose and 3% croscarmellose sodium, and mix well.
[0185] 1.4 Granulation
[0186] Add 50% ethanol solution containing 10% PVP to the above mixture to make soft material, ...
Embodiment 1
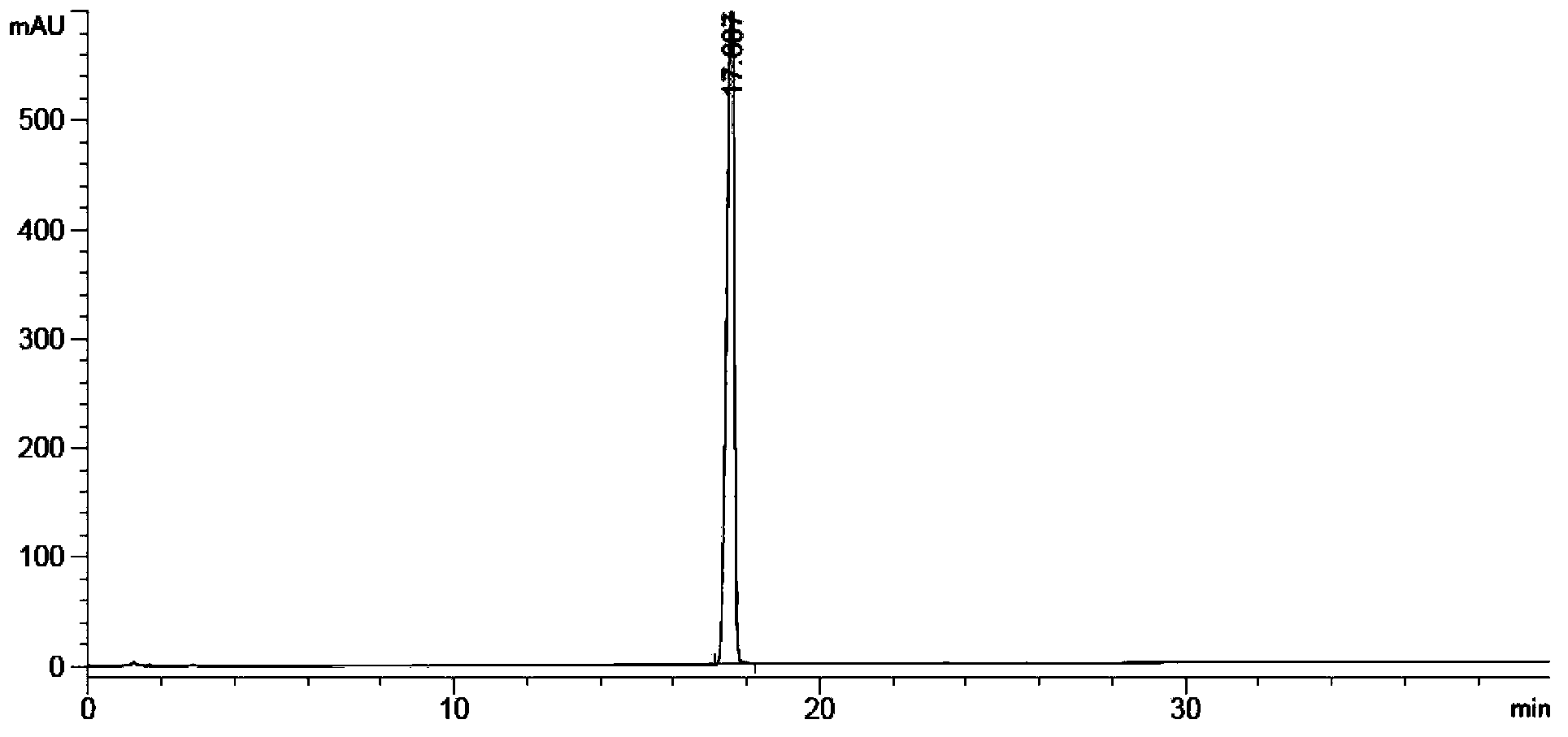
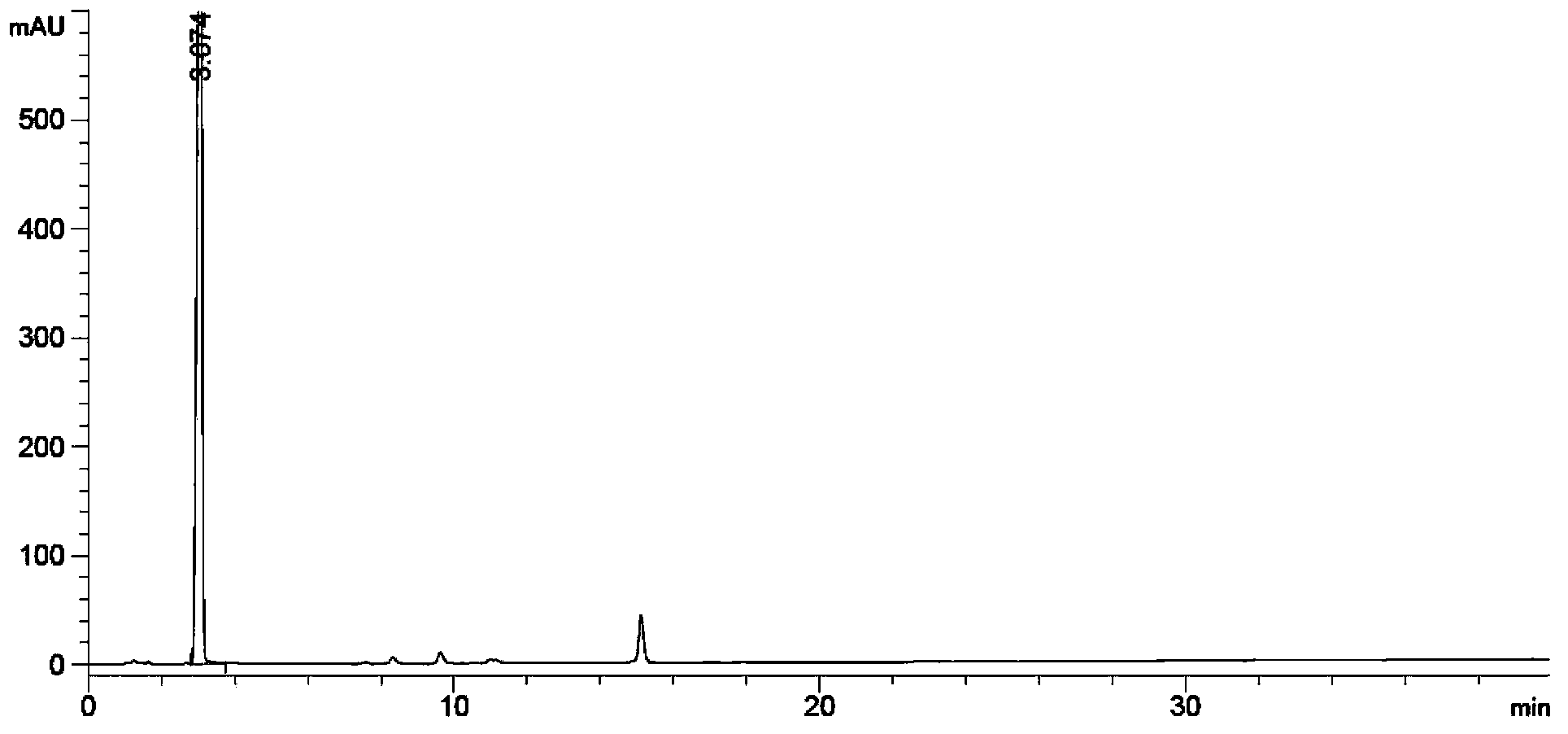
[0193] Test Method: High Performance Liquid Chromatography (Appendix ⅤD of Part Two of Chinese Pharmacopoeia 2010 Edition)
[0194] Test conditions:
[0195] Mobile phase: use 0.005M sodium dihydrogen phosphate (adjust pH to 3.2 with phosphoric acid aqueous solution)-acetonitrile (volume ratio 70:30) as mobile phase A, use 0.005M sodium dihydrogen phosphate (adjust pH to 3.2 with phosphoric acid aqueous solution)-acetonitrile ( Volume ratio 10:90) as mobile phase B for gradient elution according to the table.
[0196]
[0197] UV detector: detection wavelength 215nm
[0198] Chromatographic column: phenylsilane bonded silica gel as filler (eg: Inertsil PH-3, filler particle size 3μm, length 10cm, inner diameter 4.6mm)
[0199] Flow rate: 1.0ml / min
[0200] Injection volume: 10μl
[0201] Specific test operation:
[0202] Preparation of system suitability solution Accurately weigh Abiraterone acetate, A0, A1, A2, A3,
[0203] A2 acetylate, A1 dimer, appropriate amount ...
Embodiment 2
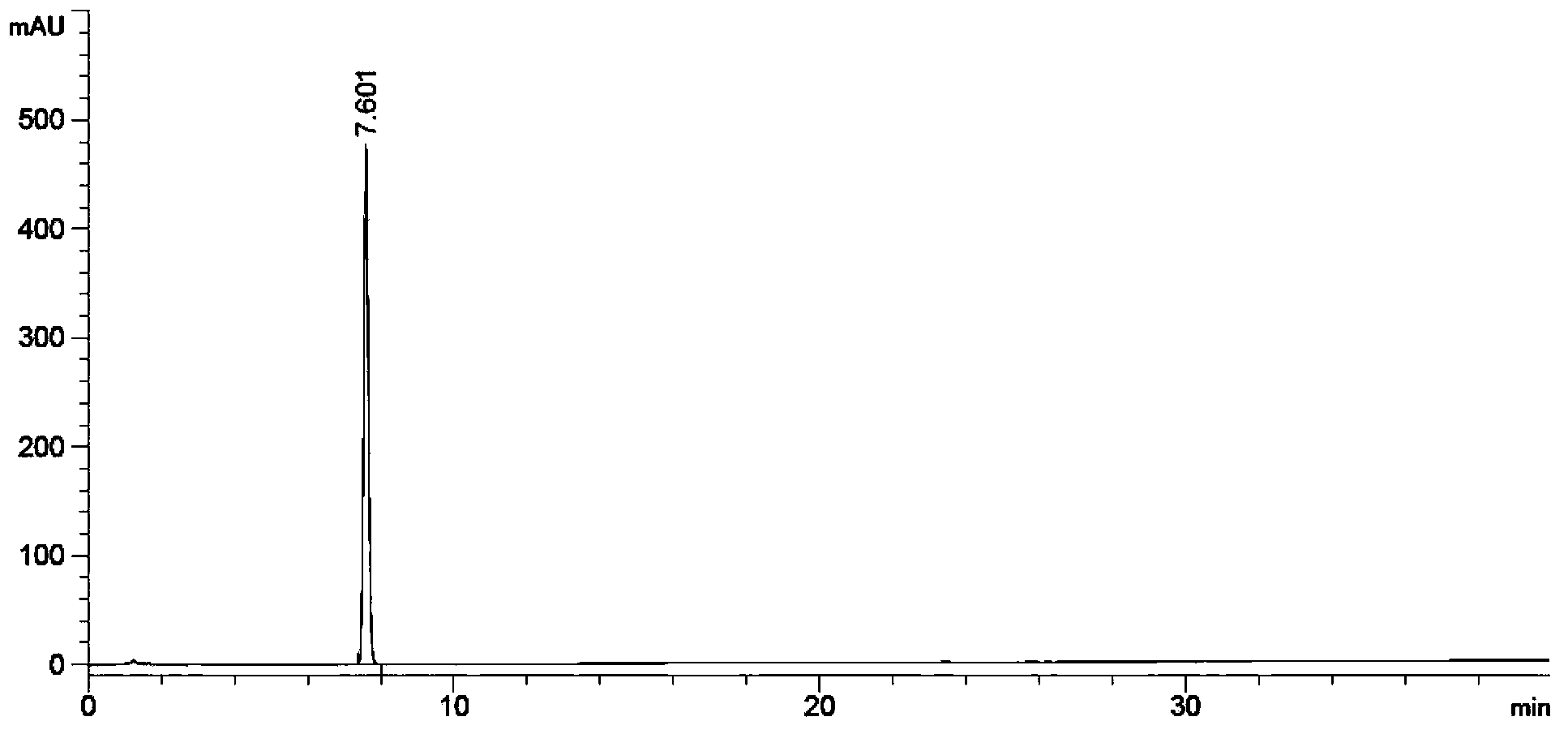
[0210] Test Method: High Performance Liquid Chromatography (Appendix ⅤD of Part Two of Chinese Pharmacopoeia 2010 Edition)
[0211] Test conditions:
[0212] Mobile phase: use 0.005M sodium dihydrogen phosphate (adjust pH to 3.2 with phosphoric acid aqueous solution)-acetonitrile (70:30) as mobile phase A, use 0.005M sodium dihydrogen phosphate (adjust pH to 3.2 with phosphoric acid aqueous solution)-acetonitrile (10: 90) Use mobile phase B to perform gradient elution according to the table below.
[0213]
[0214] UV detector: detection wavelength 215nm
[0215] Chromatographic column: phenylsilane bonded silica gel as filler (eg: Inertsil PH-3, filler particle size 3μm, length 10cm, inner diameter 4.6mm)
[0216] Flow rate: 1.0ml / min
[0217] Injection volume: 10μl
[0218] Specific test operation:
[0219] Preparation of system suitability solution
[0220] Precisely weigh the appropriate amount of abiraterone acetate, A0, A1, A2, A3, A2 acetylate, A1 dimer, acetyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com