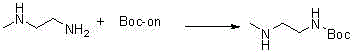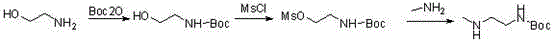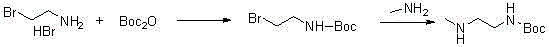Synthesis method of tert-butyl 2-(methylamino)ethylcarbamate
A technology of ethyl carbamate and methylamino, which is applied in the field of synthesis of tert-butyl 2-(methylamino)ethyl carbamate, can solve the problems of high cost of precious metal catalysts, unfavorable industrial production, low overall yield and the like , to achieve the effect of facilitating industrial production, reducing the amount of wastewater treatment, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 2-(N-isobutoxycarboxamido) ethyl isocyanate, the specific steps are as follows:
[0027] In a 1000ml three-neck flask (with water separator), N-Boc-ethylenediamine (160g, 1mol) and paraformaldehyde (31.5g, 0.35mol) were dissolved in toluene (500ml), and acetic acid (0.3g ), heated to reflux for 5 hours until no more water was separated from the water separator. Then, the reaction solution was concentrated to remove the solvent, the residue was dissolved in ethyl acetate (500ml), washed with water (300ml), saturated aqueous sodium carbonate solution (300ml) and saturated brine (300ml) successively, and the organic phase was dried, Concentrate to obtain product 108 g oily liquid, yield 62%.
Embodiment 2
[0029] Preparation of 2-(N-isobutoxycarboxamido) ethyl isocyanate, the specific steps are as follows:
[0030] In a 1000ml three-necked flask (with water separator), N-Boc-ethylenediamine (160g, 1mol) and paraformaldehyde (31.5g, 0.35mol) were dissolved in benzene (500ml), and p-toluenesulfonic acid was added dropwise (0.45g), heated to reflux for 5.5 hours until no more water was separated in the water separator. Then, the reaction solution was concentrated to remove the solvent, the residue was dissolved in ethyl acetate (500ml), washed with water (300ml), saturated aqueous sodium carbonate solution (300ml) and saturated brine (300ml) successively, and the organic phase was dried, Concentrate to obtain product 113 g oily liquid, yield 65%.
[0031] Examples 1 and 2 can be repeated according to actual needs to obtain more 2-(N-isobutoxycarboxamido)ethyl isocyanide for subsequent examples.
Embodiment 3
[0033] Prepare tert-butyl 2-(methylamino)ethyl carbamate, the specific steps are as follows:
[0034] In a 1000ml three-necked flask, 2-(N-isobutoxycarboxamido) ethyl isocyanate (172 g, 1mol) was dissolved in tetrahydrofuran (600ml), at room temperature (about 20°C~30°C) Add sodium borohydride (74 g, 2mol) in batches, after the addition is complete, stir at room temperature for 4 hours, add acetic acid to quench unreacted sodium borohydride, concentrate to remove tetrahydrofuran, add water (500ml), ethyl acetate (500ml×2 ) extraction, the organic phases were combined and washed with saturated brine (500ml), the organic phase was dried and concentrated to obtain an oily crude product, which was distilled under reduced pressure by an oil pump to obtain 148 g of a colorless oily product with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com