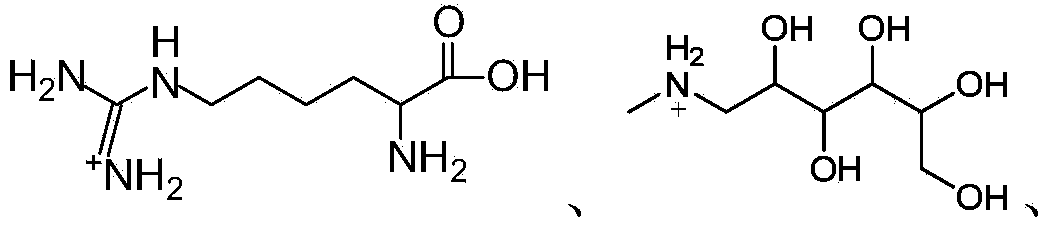
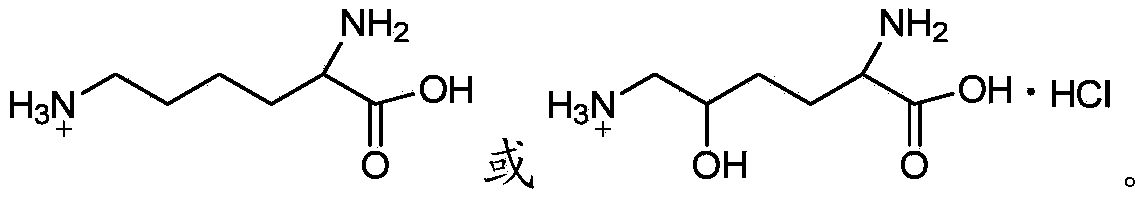
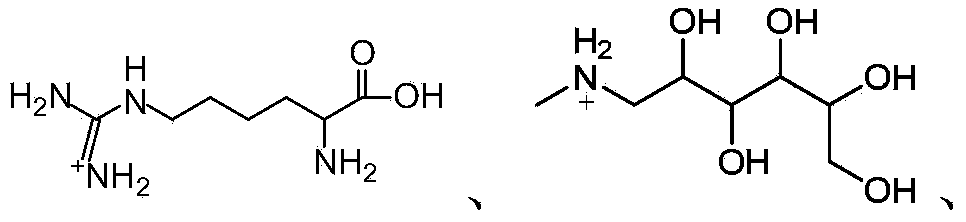
Apigenin-7-O-beta-D-glucuronide derivative, and preparation method and application thereof
A technology of scutellarin and its derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of limiting the application of scutellarin, short oral half-life, poor water solubility and fat solubility, etc. Achieve the effect of high inhibition rate, simple process and high physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of scutellarin sodium salt (structural compound shown in formula I-a):
[0071] Add 2 g (4 mmol) of scutellarin to a 250 mL round-bottomed flask, and then sequentially add 40 mL of pure water and 40 mL of acetone. Slowly add 1 mol / L sodium hydroxide aqueous solution dropwise under stirring at 0°C until the pH is 8, and the reaction solution continues to stir for half an hour until the reaction is complete. The reaction solution was filtered with a microporous membrane, about 100 mL of acetone was added to the filtrate, and a large amount of precipitation was precipitated. Continue to stir for half an hour and then filter with suction, wash the filter cake with acetone until the filtrate is colorless. The filter cake was collected and dried under reduced pressure at 30° C. to 40° C. to obtain 2 g of orange-red scutellarin sodium salt with a yield of 95% and a chromatographic purity of over 99%.
[0072] 1 HNMR (500MHz, DMSO), δ (ppm): 7.9...
Embodiment 2
[0078] Embodiment 2: the preparation of scutellarin potassium salt (structural compound shown in formula I-b)
[0079] Add 2 g (4 mmol) of scutellarin to a 250 mL round bottom flask, then sequentially add 40 mL of pure water and 20 mL of tetrahydrofuran, and slowly add 2 mol / L potassium hydroxide solution dropwise under stirring at -10°C until the pH is 9. The reaction solution was stirred for half an hour until the reaction was complete. The reaction solution was filtered with a microporous membrane, and about 100 ml of tetrahydrofuran was added to the filtrate, and a large amount of precipitation was precipitated. Continue to stir for half an hour and then filter with suction, wash the filter cake with acetone until the filtrate is colorless. The filter cake was collected and dried under reduced pressure at 30-40°C to obtain 2.1 g of orange-yellow scutellarin potassium salt with a yield of 96% and a chromatographic purity of over 99%.
[0080] 1 HNMR (500MHz, DMSO), δ (pp...
Embodiment 3
[0086] Embodiment 3: the preparation of scutellarin lithium salt (structural compound shown in formula I-c)
[0087] Add 2 g (4 mmol) of scutellarin to a 250 mL round-bottomed flask, and then sequentially add 40 mL of pure water and 80 mL of acetonitrile. Under magnetic stirring at 30°C, slowly add 3 mol / L lithium hydroxide aqueous solution dropwise until the pH is 8.5, and continue stirring for half an hour until the reaction is complete. The reaction solution was filtered with a microporous membrane, and about 100 ml of acetonitrile was added to the filtrate, and a large amount of precipitation was precipitated. Continue to stir for half an hour and then filter with suction, wash the filter cake with acetone until the filtrate is colorless. The filter cake was collected and dried under reduced pressure at 30-40° C. to obtain 1.9 g of orange-yellow scutellarin lithium salt with a yield of 95% and a chromatographic purity of over 99%.
[0088] 1 HNMR (500MHz, DMSO), δ (ppm)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com