Artificial antigen and kit for combined detection of eb virus rta protein antibody and eb virus early antigen ea antibody
A technology of Epstein-Barr virus and early antigen, applied in the field of medical immunology, in vitro serological diagnosis, and biotechnology, can solve the problems of poor sensitivity, poor specificity, and high missed detection rate of nasopharyngeal carcinoma, and achieve low cost, easy operation, and shortened time. The effect of detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
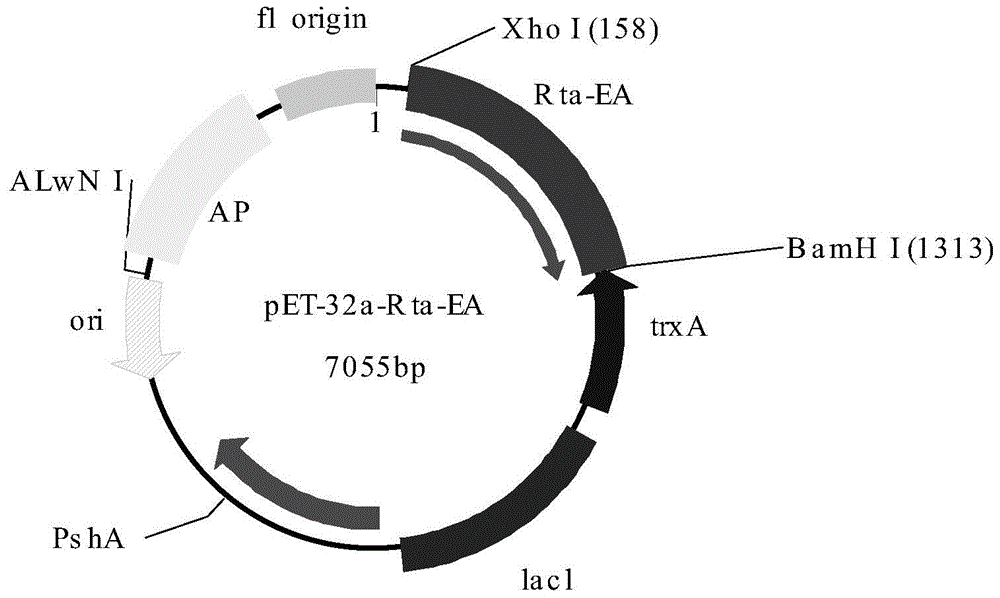
[0046] Example 1 Tandem expression of Epstein-Barr virus Rta protein and EA antigen in Escherichia coli
[0047] Look up the amino acid sequence of Epstein-Barr virus Rta protein, EA antigen (see sequence table SEQ ID NO:7 and SEQ ID NO:8) on NCBI website, adopt http: / / www.imtech.res.in / raghava / bcepred / bcepred_submission. html analyzes its epitope, selects its epitope, obtains the optimized Epstein-Barr virus Rta protein fragment (Seq ID No: 1) and the optimized Epstein-Barr virus EA antigen (Seq ID No: 2), and codes sub for optimization. The optimized Rta and EA antigen gene sequences were combined and synthesized, and primers were designed with Primer5.0 primer design software.
[0048] Upstream primer P1: 5'-CCTCGAGATGGCTTCTTCTAACCGT-3',
[0049] Downstream primer P2: 5'-CTGGATCCTTTGGTTTTGAAACGCAG-3'.
[0050] P1 and P2 primers respectively contain Xho I and BarmH I restriction sites.
[0051] The above synthetic gene was amplified by PCR technique.
[0052] PCR reacti...
Embodiment 2
[0057] Example 2 Preparation of a kit for joint detection of Epstein-Barr virus Rta protein antibody and early antigen EA antibody
[0058] (1) Composition of the kit
[0059] 1. ELISA plate coated with Rta-EA antigen: the coating protein is high-purity recombinant Rta-EA protein, and the coating concentration can be 0.05-0.25 μg / ml;
[0060] 2. The working dilution of HRP-labeled goat anti-human IgA can be 1:5000—1:80000;
[0061] 3. Calibrator solution, positive control solution, negative control solution, 1 bottle each, 1ml / bottle;
[0062] 4. Sample diluent: 50mM phosphate buffer containing 0.3M NaCl, 25ml / bottle, 2 bottles;
[0063] 5. Substrate chromogenic solution: composed of A liquid and B liquid, the substrate chromogenic liquid A is carbamide peroxide, 7ml / bottle, 1 bottle; the substrate chromogenic liquid B is tetramethylbenzidine, 7ml / bottle, 1 bottle;
[0064] 6. Termination solution: 2mol / l sulfuric acid;
[0065] 7. Concentrated washing solution: pH valu...
Embodiment 3
[0081] Embodiment 3 Clinical trial evaluation of kit of the present invention
[0082] 55 normal human samples and 58 patient samples diagnosed with nasopharyngeal carcinoma were detected with the kit prepared by the present invention. The detection procedure and result judgment were carried out in strict accordance with the usage method of the kit provided in Example 2, and VCA antibody was selected for detection at the same time. The kit and the EA antibody detection kit (both commercially purchased from the Chinese Institute of Preventive Medicine and Virology) were tested in parallel, and the specific operations were performed according to the instructions of the kits. The test results are shown in Table 1.
[0083] Table 1 Comparison of test results
[0084]
[0085] It can be seen from the above results that the kit provided by the present invention has excellent performance in the diagnosis and screening of nasopharyngeal carcinoma, with a sensitivity of 91.4% and a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com