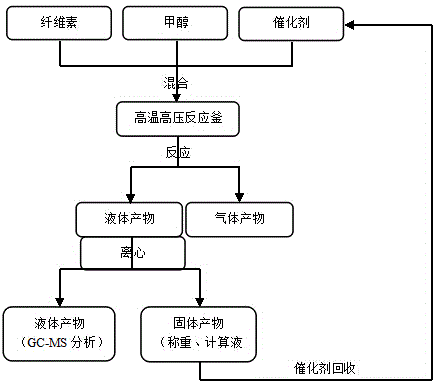
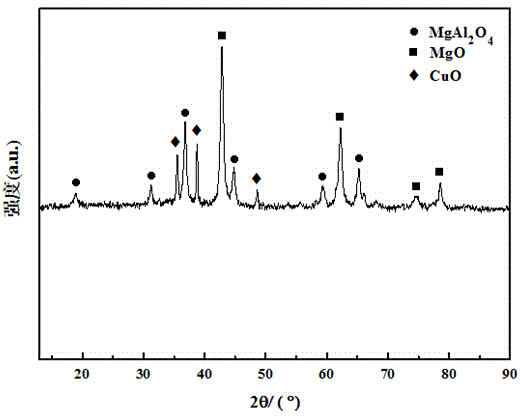
Catalyst preparation method and application
A catalyst and catalytic liquefaction technology, applied in the field of catalytic chemistry, can solve the problems of high price, poor catalyst structure and activity stability, and achieve high liquefaction rate, good stability and good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
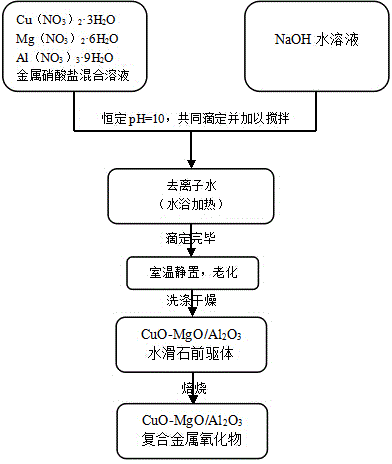
[0036] CuO-MgO-Al described in this example 2 o 3 The preparation method of catalyst specifically comprises the following steps:
[0037] (1) Three metal nitrates Cu (NO 3 ) 2 ·3H 2 O, Mg (NO 3 ) 2 ·6H 2 O and Al (NO 3 ) 3 9H 2 O is dissolved in deionized water to obtain a metal nitrate mixed solution, wherein Cu in the metal nitrate mixed solution 2+The molar concentration is 1.5mol / L, Mg 2+ The molar concentration is 1.0mol / L, Al 3+ The molar concentration is 2.5mol / L;
[0038] (2) Using the constant pH co-precipitation method, pH=10, drop the metal nitrate mixed solution obtained in step (1) and the NaOH solution with a molar concentration of 1.0mol / L into constant temperature deionized water, and stir to make it Mix evenly, wherein, deionized water is placed in a constant temperature water bath, and the temperature of the water bath is 65°C; the volume ratio of NaOH solution and metal nitrate mixed solution is 1:2, and the stirring speed is 300r / min;
[0039]...
Embodiment 2
[0047] CuO-MgO-Al described in this example 2 o 3 Catalyst preparation methods, such as figure 1 As shown, it specifically includes the following steps:
[0048] (1) Three metal nitrates Cu (NO 3 ) 2 ·3H 2 O, Mg (NO 3 ) 2 ·6H 2 O and Al (NO 3 ) 3 9H 2 O was dissolved in deionized water to obtain a metal nitrate mixed solution, and in the metal nitrate mixed solution Cu 2+ The molar concentration is 0.5mol / L, Mg 2+ The molar concentration is 1.0mol / L, Al 3+ The molar concentration is 1.5mol / L, Cu 2+ and Mg 2+ The molar concentration ratio is 0.5:1, Cu 2+ and Mg 2+ The sum of molar concentrations and Al 3+ The molar concentration ratio is 1:1;
[0049] (2) Using the constant pH co-precipitation method, pH=10, drop the metal nitrate mixed solution obtained in step (1) and the NaOH solution with a molar concentration of 1.0mol / L into constant temperature deionized water, and stir to make it Mix evenly, wherein, deionized water is placed in a constant temperature...
Embodiment 3
[0055] CuO-MgO-Al described in this example 2 o 3 The preparation method of catalyst specifically comprises the following steps:
[0056] (1) Three metal nitrates Cu (NO 3 ) 2 ·3H 2 O, Mg (NO 3 ) 2 ·6H 2 O and Al (NO 3 ) 3 9H 2 O is dissolved in deionized water to obtain a metal nitrate mixed solution, wherein Cu in the metal nitrate mixed solution 2+ The molar concentration is 2.5mol / L, Mg 2+ The molar concentration is 2.5mol / L, Al 3+ The molar concentration is 5.0mol / L;
[0057] (2) Using the constant pH co-precipitation method, pH=10, drop the metal nitrate mixed solution obtained in step (1) and the NaOH solution with a molar concentration of 0.5mol / L into constant temperature deionized water, and stir to make it Mix evenly, wherein, deionized water is placed in a constant temperature water bath, and the temperature of the water bath is 100°C; the volume ratio of NaOH solution and metal nitrate mixed solution is 1:1, and the stirring speed is 500r / min;
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com