Method for preparing HCFC-244bb
A technology of tetrafluoropropane and trifluoropropene, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor reaction effect and complicated process, and achieve stable performance, simple process and good reaction effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
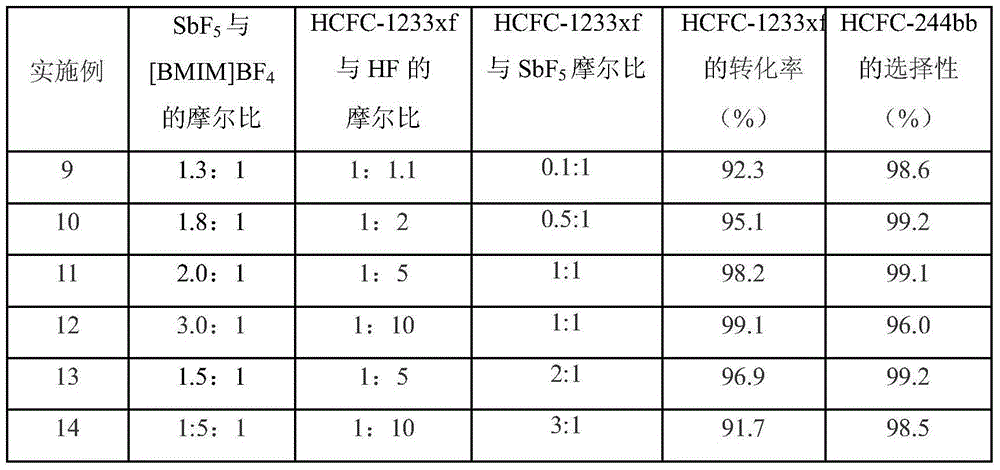
[0012] The liquid phase fluorination reaction was carried out in a stirred 200mL stainless steel autoclave. Add 15g of SbF to the reactor in sequence 5 , 10.1g [BMIM]BF 4 , then add 5g HF, stir for 4h, raise the temperature to 50°C, and keep the temperature constant for 1h.
[0013] Add 13g of HCFO-1233xf into the reactor, react at 50°C for 1 hour and then lower the temperature. The reaction product was analyzed by gas chromatography after being washed with water to remove acid, and the conversion rate of HCFO-1233xf was 95.3%, and the selectivity of HCFC-244bb was 99.1%.
Embodiment 2
[0015] The liquid phase fluorination reaction was carried out in a stirred 200mL stainless steel autoclave. Add 20.8g SbCl to the reactor successively 5 , slowly inject 30g HF for fluorination treatment, control the pressure within 0.20MPa during the treatment process, raise the temperature to 70°C, keep the temperature for 2h, add 10.1g [BMIM]BF 4 , constant temperature 4h.
[0016] Add 13g of HCFO-1233xf into the reactor, the reaction temperature is 70°C, and cool down after 3 hours of reaction. The reaction product was analyzed by gas chromatography after being washed with water to remove acid, and the conversion rate of HCFO-1233xf was 90.3%, and the selectivity of HCFC-244bb was 98.6%.
Embodiment 3~5
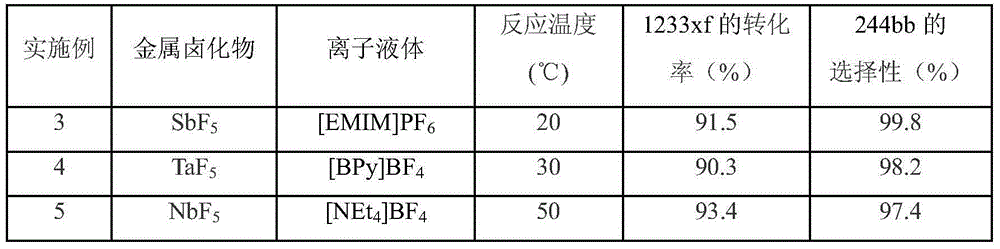
[0018] Examples 4-6 The method for preparing HCFC-244bb is the same as that of Example 1, except that the metal halide, the type of ionic liquid and the reaction temperature are changed. The reaction results are shown in Table 2.
[0019] Table 2
[0020]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com