Heavy metal ion adsorbent ferrite hollow spheres MFe2O4
A technology of ferrite hollow spheres and heavy metal ions, which is applied in the fields of environmental protection and environmental water treatment, can solve the problems of limited maximum adsorption capacity, and achieve good application and promotion value, high controllability, and strong adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1—4
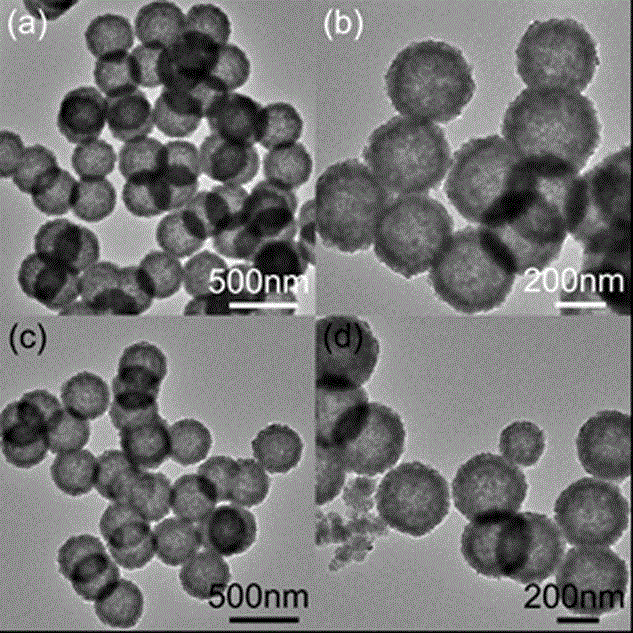
[0032] Ferrite Hollow Ball FeFe 2 o 4 The preparation method of adsorbent comprises the following steps:
[0033] (1) Dissolve 1.5 mmol (0.567 g) of ferric chloride hexahydrate, 3 mmol (0.612 g) of trisodium citrate dihydrate, and 4.5 mmol (0.345 g) of anhydrous sodium acetate in 30 ml of deionized water to form a a light green transparent solution;
[0034] (2) Add 0.3g PAM (polyacrylamide) to the solution in step (1), and stir vigorously for 30 minutes until PAM is completely dissolved;
[0035] (3) Transfer the solution in step (2) to a reaction kettle with a capacity of 40mL, react at 180°C for 12h, and cool to room temperature after the reaction;
[0036] (4) Wash the sample obtained in step (3) with deionized water and ethanol, and dry it in vacuum at 60°C for 6 h to obtain the micronano-structured ferrite hollow sphere FeFe 2 o 4 (i.e. ferric oxide Fe 3 o 4 ).
Embodiment 2
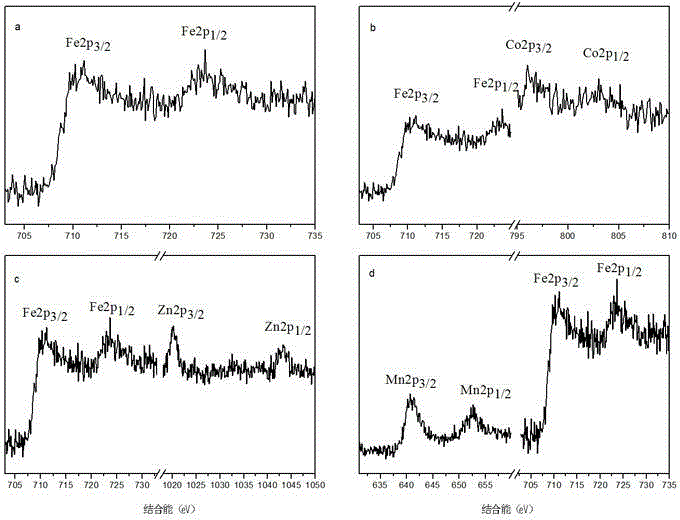
[0037] It should be noted that by changing the 1.5 mmol ferric chloride hexahydrate raw material in step (1) to 1.0 mmol (0.378 g) ferric chloride hexahydrate and 0.5 mmol (0.173 g) cobalt chloride hexahydrate, iron can be prepared Cobalt acid hollow sphere CoFe 2 o 4 , which is Example 2 ;
[0038] Change the raw material of 1.5 mmol ferric chloride hexahydrate in step (1) to 1.0 mmol (0.378 g) ferric chloride hexahydrate and 0.5 mmol (0.068 g) zinc chloride to prepare zinc ferrite hollow spheres Zn Fe 2 o 4 , which is Example 3 ;
[0039] Change the raw material of 1.5 mmol ferric chloride hexahydrate in step (1) to 1.0 mmol (0.378 g) ferric chloride hexahydrate and 0.5 mmol (0.0985 g) manganese chloride tetrahydrate to prepare manganese ferrite hollow spheres MnFe 2 o 4 , which is Example 4 .
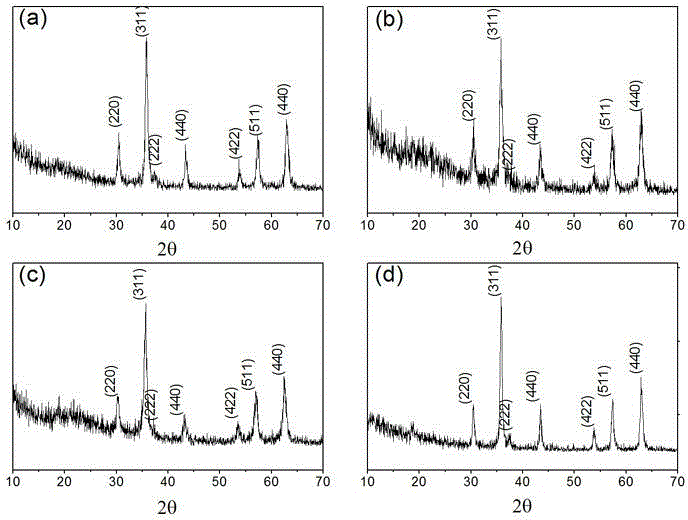
[0040] For the prepared Fe 3 o 4 (Example 1), CoFe 2 o 4 (Example 2), ZnFe 2 o 4 (Example 3), MnFe 2 o 4 (Example 4) The XRD collection of patterns sees figure 1...
experiment example
[0046] In order to detect the actual adsorption effect of the ferrite hollow sphere adsorbent provided by the present invention on hexavalent chromium ions or pentavalent arsenic ions, the inventor has done a further detection experiment, and the experimental process is briefly described as follows:
[0047] During the experiment, the amount of ferrite hollow sphere adsorbent was 10 mg / L.
[0048] During the experiment, the simulated wastewater was a heavy metal ion solution prepared with sodium arsenate and potassium dichromate, composed of NaOH solution and HNO 3 The solution adjusts the pH value of the heavy metal ion solution.
[0049] In the specific experiment, the ferrite hollow spheres and the simulated wastewater were placed in a plastic conical flask at room temperature. During the adsorption process, the conical flask was placed in a 250 n / mim ultrasonic instrument to disperse the adsorbent evenly until the adsorption was achieved. Equilibrium: After adsorption equ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com