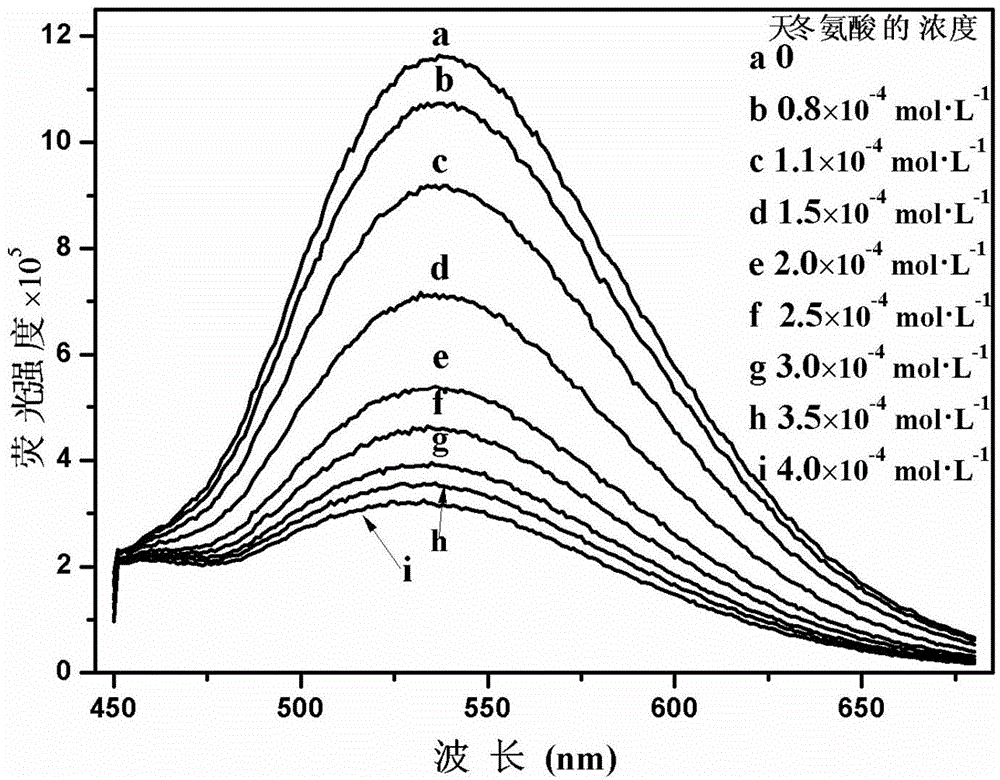
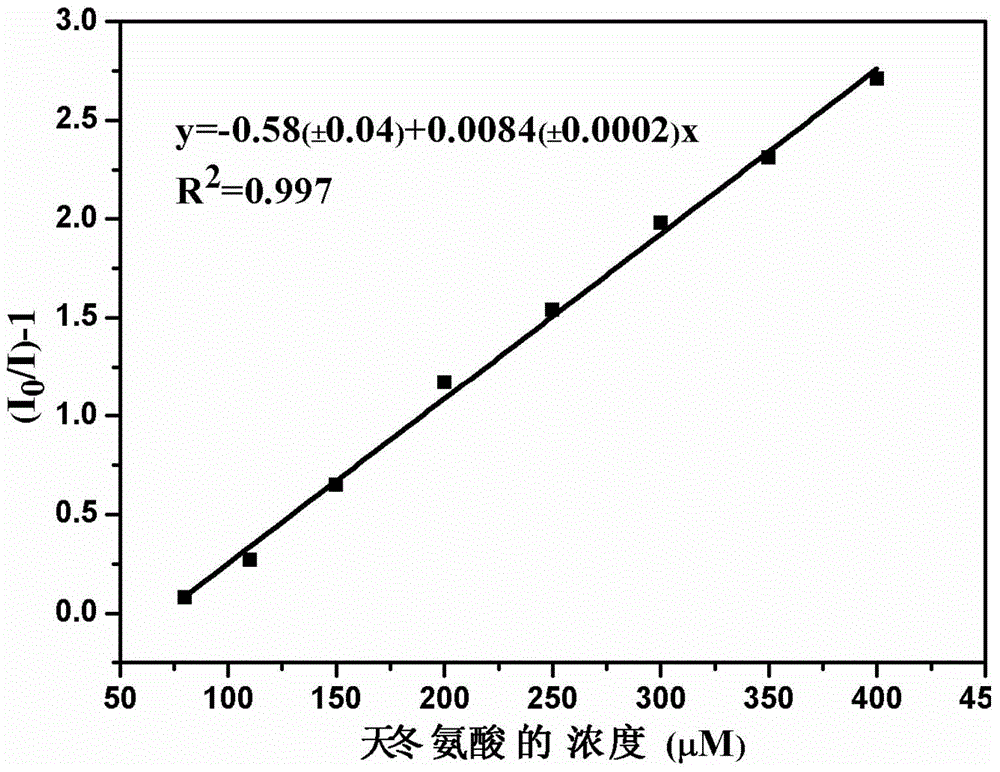
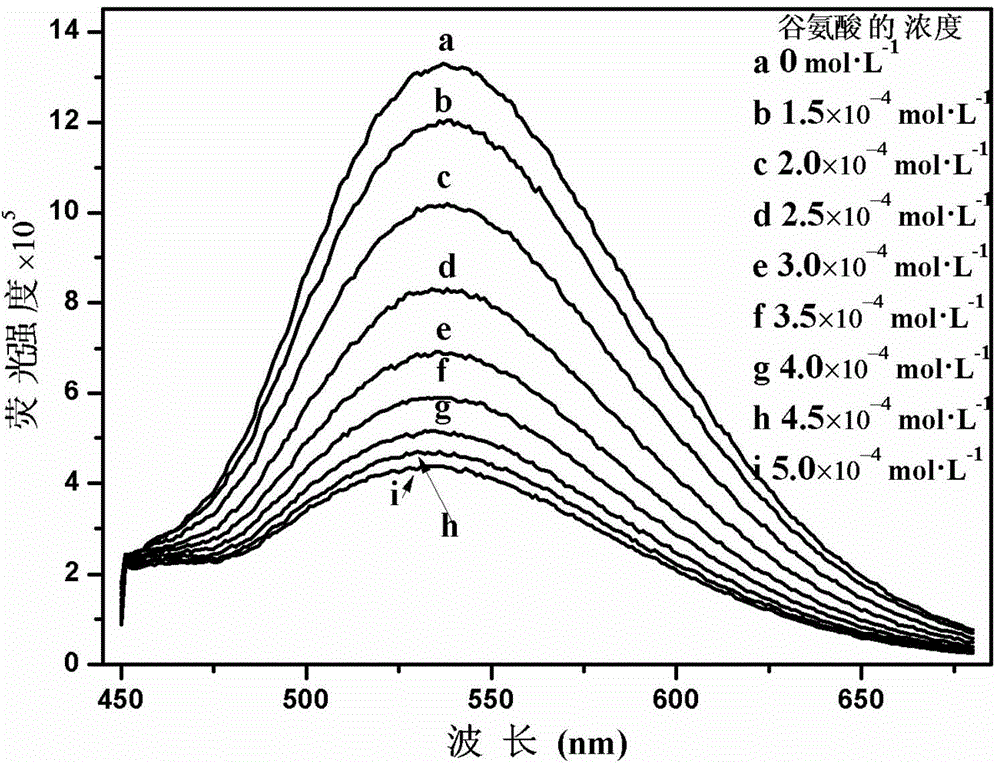
Cationic fluorescent probe for dansyl biimidazole derivatives as well as synthesis method and application of cationic fluorescent probe
A technology of bisimidazole dansyl derivatives and diethylenetriamine dansyl derivatives, which is applied in the field of fluorescent probes and their synthesis, can solve the problems of large errors, long analysis time, and low sensitivity, and achieve low detection Limit detection, good chemical stability, and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Synthesis of diethylenetriamine protected by tert-butoxycarbonyl groups at both ends
[0036] Add 1mL (9.3mmol) diethylenetriamine, 4.0mL (22mmol) tert-butylphenyl carbonate, 0.3mL (2.2mmol) ) triethylamine (TEA), stirred for 12 hours under nitrogen protection and ice-bath conditions, the reaction solution was poured into 1L0.025mol / L K 2 HPO 4 and 0.025mol / L NaH 2 PO 4 In the buffer solution, use 2mol / L H 2 SO 4Adjust the pH of the solution to 3, then extract with ether, take the water layer, adjust the pH of the water layer to 10 with 9mol / L NaOH aqueous solution, extract three times with dichloromethane, take the dichloromethane layer, wash with anhydrous sodium sulfate Drying, dichloromethane is distilled off by rotary evaporation to obtain the diethylenetriamine protected by tert-butoxycarbonyl groups at both ends shown in formula I. The reaction equation is as follows:
[0037]
[0038] 2. Synthesis of diethylenetriamine dansyl derivatives protected by...
Embodiment 2
[0051] In step 2 of the synthesis of diethylenetriamine dansyl derivatives protected by both ends of the tert-butoxycarbonyl group in Example 1, 2.0 g (7 mmol) of dansyl chloride and 3.4 mL (24.5 mmol) of triethylamine were added to a container containing In a three-necked flask of 70 mL of chloroform, stir and dissolve, then add 2.1 g (7 mmol) of diethylenetriamine protected by tert-butoxycarbonyl groups at both ends, and stir for 12 hours under nitrogen protection and normal temperature conditions. Other steps are the same as in Example 1. Similarly, a cationic fluorescent probe of the bis-imidazole dansyl derivative was obtained with a yield of 38%.
Embodiment 3
[0053] In the step 4 of the synthesis of diethylenetriamine dansyl derivatives with brominated ends in Example 1, 0.25g (0.7mmol) of diethylenetriamine dansyl derivatives with amino groups at both ends and 0.4mL (18mmol) three Ethylamine was added to a three-necked flask containing 50 mL of chloroform, stirred and dissolved, and 0.176 mL (1.75 mmol) of bromoacetyl bromide and 12 mL of chloroform were placed in a constant-pressure dropping funnel, under nitrogen protection and ice bath conditions. Add it dropwise into a three-necked flask and stir for 48 hours. Other steps are the same as in Example 1 to obtain a cationic fluorescent probe of bis-imidazolyl dansyl derivative with a yield of 59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com